Post-traumatic lung injury may lead to acute respiratory distress syndrome (ARDS).1-2 Non invasive ventilation (NIV) has been used both before and after extubation in these patients.1-8 The sequential use of NIV and High Flow Nasal Therapy (HFNT)9-11 may improve oxygenation while avoiding intubation, preventing reintubation, and reducing the length of invasive mechanical ventilation (iMV).12,13 However, there is scarce evidence on the use of HFNT the trauma population at risk of weaning failure.14
This prospective single-center pilot study assessed feasibility and safety of early extubation.15,16 followed by sequential use of NIV and HFNT in consecutive blunt chest trauma patients admitted to ICU between January 2nd 2017 and December 31st 2019. The study was approved by the local Ethics Committee and registered. All patients or legal representatives gave their written permission.
Patients were included for early extubation14 if meeting all the following criteria: 1) age ≥18 years; 2) invasive Mechanical Ventilation (iMV) for at least 48 h; 3) pressure support ventilation (PSV) with a total applied pressure (i.e. positive end-expiratory pressure (PEEP) + inspiratory support) ≤20 cmH2O and a PEEP level between 8 and 10 cmH2O; 4) the ratio between the partial pressure of oxygen and fraction of inspired oxygen (PaO2/FiO2 ratio) between 200 and 300 mmHg with FiO2 ≤ 0.6 and lack of ARDS; 5) PaCO2 ≤ 45 mmHg and pH ≥7.35; 6) respiratory rate (RR) ≤ 30 breaths/min; 7) core temperature <38.5 °C; 8) Glasgow Coma Scale (GCS) ≥11; 9) Richmond Agitation Sedation Score (RASS) <3; 10) preserved cough-reflex on suctioning and need for <2 tracheobronchial suctioning per hour.
Patients were excluded if they met one or more of the following criteria: 1) hemodynamic instability (defined as systolic arterial pressure <90 mmHg despite fluid resuscitation and/or use of vasopressors); 2) life-threatening arrhythmias and/or electrocardiographic signs of ischemia; 3) sepsis; 4) secondary acute respiratory failure due to neurological disorders, status asthmaticus, chronic obstructive pulmonary disease or cardiogenic pulmonary edema; 5) tracheotomy; 6) uncontrolled vomiting; 7) RASS ≥3; 8) two or more organ failures; 9) body mass index (BMI) > 30 kg/m; 210) documented history or suspicion of obstructive sleep apnea; 11) unstable flail chest; 12) recent upper airway or esophageal surgery; 13) pregnancy; 14) inclusion in other research protocols; 15) denied consent.
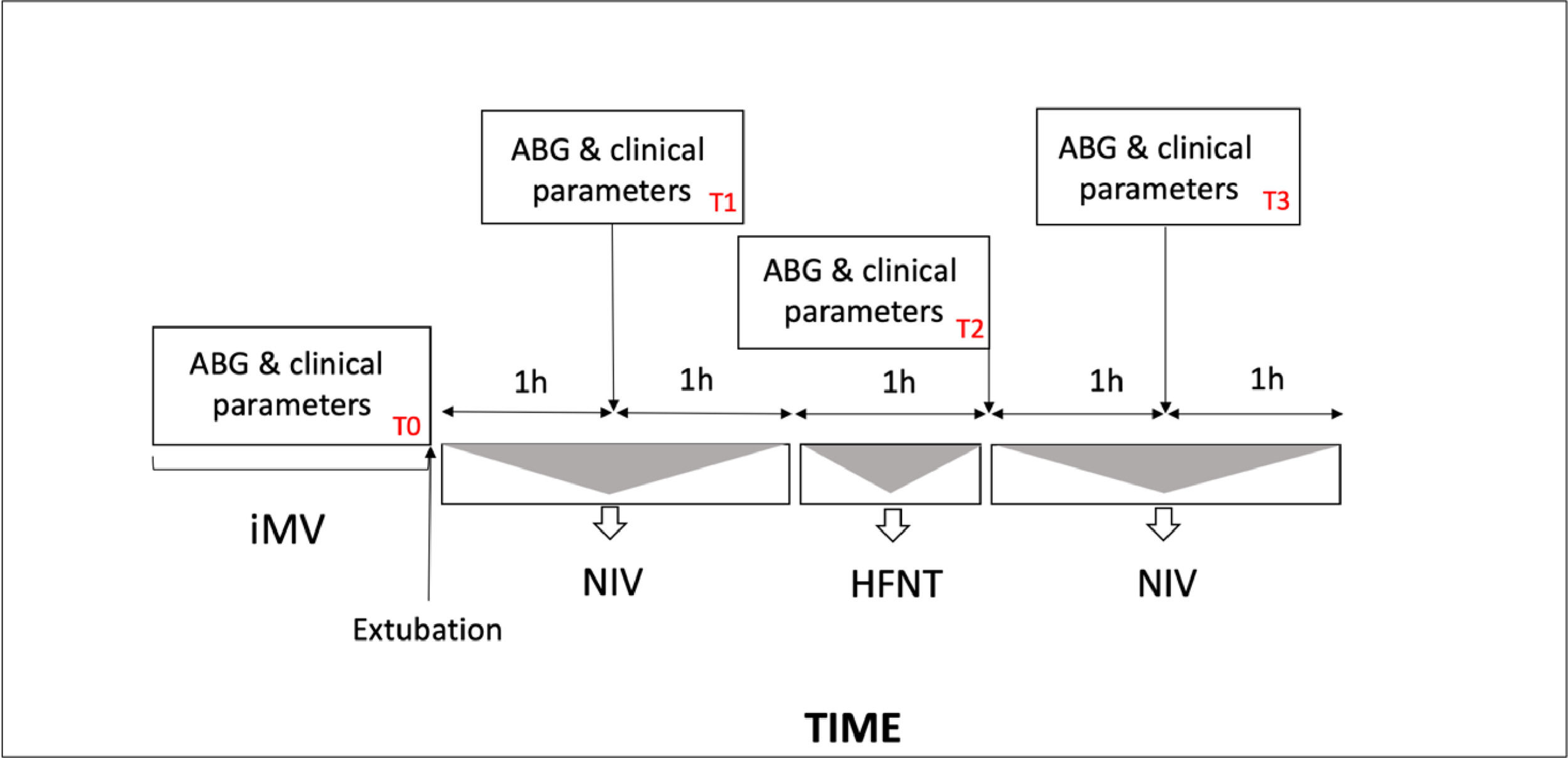
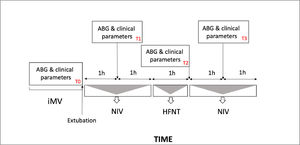
Patients underwent sequential application of NIV and HFNT as represented in Fig. 1. During NIV session full-face or oronasal masks were rotated to avoid skin breakdown.17 Humidification was achieved through a heated humidifier. Non invasive pressure support ventilation (PSV) was set up according to the pressure level applied before extubation and was then titrated to achieve an expired tidal volume of 7–8 ml/kg, with PaCO2 < 45 mmHg, pH >7.35, and respiratory rate (RR)< 30 breaths/min. PEEP was adjusted to maintain PaO2/FiO2 ratio > 225 mmHg.
Protocol flowchart and study times point. (T0), before extubation; (T1), 1 h after extubation during the first NIV cycle, (T2), 3 h after extubation during the first HFNT session; (T3) 4 h after extubation during second NIV cycle. iMV, invasive mechanical ventilation, NIV noninvasive mechanical ventilation, HFNT high flow nasal therapy.
HFNT was delivered at a gas flow rate of 60 L/min and FIO2 of 1.0, via large-bore dedicated nasal prongs at 37 °C and then adjusted according to the patient's comfort. FiO2 was then adjusted to maintain a SpO2 > 92%.
After extubation patients received intravenous analgo-sedation with remifentanil (dose range of 0.02–0.08 mcg/kg/min) or dexmetedomidine (dose range 0.4–1 mcg/kg/h) in case of RASS ≥1 and/or Behavioural Pain Scale (BPS) >4. For lower BPS levels acetaminophen 1 g three time a day was given.
A weaning trial was attempted when patients reached PaO2/FiO2 ratio > 250 mmHg. In these cases, PSV levels and PEEP were progressively decreased (by 2 cmH2O sequential steps), until they reached a minimum threshold value of 5 and 8 cmH2O, respectively. Then, NIV was interrupted, and patients were switched to HFNT only. Weaning from HFNT was considered successful if patients met all the following criteria: pH >7.35, PaCO2〈 45 mmHg and PaO2〉 70 mmHg, RR < 30 breaths/min, absence of dyspnea, respiratory accessory muscles recruitment, and paradoxical abdominal motion during 30-min trial with oxygen supplementation through a Venturi-mask with a FiO2 0.35.
Reintubation was considered in the occurrence of any of the following complications during the study period: a) cardiac or respiratory arrest; b) inability to protect the airway; c) coma or psychomotor agitation with RASS >3 not controlled by continuous i.v. sedative infusion; d) unmanageable secretions or uncontrolled vomiting; e) life-threatening arrhythmias or electrocardiographic signs of ischemia; f) hemodynamic instability; g) intolerance to all interface; or in the occurrence of at least two of the following criteria: h) PaO2/FiO2 ratio < 200 mmHg; i) respiratory acidosis (pH < 7.35 and PaCO2 > 50 mmHg); j) RR > 40 breaths /min. Vital parameters (systemic blood pressure - SBP, heart rate - HR, respiratory rate -RR) were continuously monitored.
Continuous variables were expressed as means standard deviation (SD) or as medians (interquartile range, IQR) as appropriate, and discrete variables as counts (percentage). The analysis of the medians over the times, was carried out using the non-parametric Friedman test, considered not-normal distribution of values. Pairwise comparisons to groups were carried out using Wilcoxon signed-rank test and P-value adjustment with Bonferroni's method. The association between the presentation variables were first analyzed by mean of non-parametric Spearman correlation coefficient. Multiple linear regression was used to identify the variables with an important contribution to the response variability and to adjust for confounding variables with the stepwise method by Aikake Information Criterion (Aic). A P value <0.05 was considered statistically significant.
Twenty-five patients met the inclusion criteria during the study period; five patients were excluded before intervention due to respiratory and cardiovascular complications. Baseline characteristics of the study cohort at admission are shown in Table 1a. iMV was required for a mean time of 56.4 h (55.5). The protocol, ranged from 6.5 to 100.5 h (41.4 ± 28.36). Table 1b summarizes the results of blood gas analysis and clinical parameters at different time points and at the end of the study. No cases of reintubation or death were recorded. We found that there was a positive correlation between the time of iMV and the duration of the protocol, R = 0.624 (95%; CI 0.25–0.836, p. = 0.0033). Each hour of iMV was associated with an average 32 min increase in protocol time (p. 0.005).
Baseline characteristics of the patients.
BMI: Body Mass Index, GCS: Glasgow Coma Scale, TTSs: Thoracic Trauma Severity Score.
Data are expressed by Mean (SD), Median (IQR).
Analysis of the medians (IQR) of each variables at different study times point. (T0), before extubation; (T1), 1 h after extubation during the first NIV cycle, (T2), 3 h after extubation during the first HFNT session; (T3) 4 h after extubation during second NIV cycle and at the end of treatment. NIV noninvasive ventilation, HFNT high flow nasal therapy.
Five patients, during HFNT needed to be switched to NIV because of PaO2/FiO2 ratio between 200 and 225 mmHg before reattempting HFNT. No severe adverse events were recorded. Comfort score as measured by VAS was not significantly different (p. 0.66) over time between HFNT and NIV as well as vital parameters were not different over time between HFNT and NIV. Remifentanil was never used in any patients while dexmetedomidine was used in 16 patients (mean dose range 0.8 mcg/kg/h ± 0,2).
In this prospective single-center observational pilot study of a cohort of chest trauma patients, the sequential use of NIV and HFNT following early extubation was found to be feasible and safe. None of the included patients required reintubation. Alternating the use of NIV and HFNT has proven to cause neither clinically relevant changes in respiratory parameters nor complications. This strategy may help avoid re-intubation in this patient population. However, our study has several limitations. Firstly, it lacks a control group since we did not compare the applied post-extubation protocol with other protocols or weaning practices. Secondly, we applied restrictive inclusion criteria obtaining a small, selected cohort of patients. This could explain the fact that none of the treated patients were intubated. Thirdly we did not compare dyspnea at the different study times point.
Therefore, the preliminary results obtained have low generalizability in the reference population and should be considered as feasibility, hypothesis-generating study for further controlled trials.