COPD is one of the most common pulmonary diseases and one of the leading causes of death worldwide. Exacerbations of COPD include acute worsening that could lead to hospitalization and death. In this study, our objective was to investigate the natural course of moderate and severe exacerbations (SAE) and mortality in the Hungarian population in the past decade.
MethodsA retrospective financial database analysis was performed to examine the risk of additional SAEs and death after the first ever SAE in COPD patients, using the financial database of the Hungarian National Health Insurance Fund (NHIF). Patients were enrolled between 2009.01.01. and 2019.12.31. if they had received at least one inhaled drug (LABA, LAMA, ICS or SABA/SAMA) and had been hospitalized for a COPD exacerbation (ICD-10 code J44).
ResultsA total of 63,037 patients with COPD were enrolled after their first SAE. Of them, 27,095 patients suffered at least one subsequent SAE, and 32,120 patients died during the 10-year follow-up. The median survival was 4.7 years. The risk of subsequent hospitalizations increased significantly after each SAE, with hazard ratios ranging from 1.65 to 5.01. The risk for mortality was increased after each SAE, but did not increase further with the number of SAEs. Moreover, the risk for subsequent SAE and death increased with moderate exacerbations; however, this risk did not increase further with each event.
ConclusionsDespite a relevant improvement in COPD treatment, the natural course of exacerbations remained unchanged. This result highlights the importance of preventing exacerbations and the need for more research to better predict them.
Chronic obstructive pulmonary disease (COPD) is an irreversible, long-term respiratory syndrome that, in most cases, is the result of years of cigarette smoking. The disease is characterised by a slow but progressive worsening of respiratory function and symptoms, such as cough, sputum production and dyspnoea. The slow disease progression is often interrupted by acute exacerbations (AE).1
The exact definition of AE has varied over time and between studies and working groups, but all the definitions include the basic characteristics of the event: an acute worsening of symptoms, leading to the need for a change in patient care (e.g. medication, hospitalization or even assisted ventilation).1–4 The long-term consequences of AEs are well known: worsening of respiratory symptoms, a decrease in lung function and quality of life (QoL) and an increase in the risk of hospitalization and mortality.1–5 Furthermore, it is well established that the occurrence of one AE increases the risk of another AE, especially if the first AE required hospital admission.6–8 For all the above reasons, the GOLD guidelines state that one of the key goals of COPD therapy is the prevention and appropriate treatment of AEs.1
Among the first to show the above results, Suissa et al. in their publication from 2012 reported that severe AEs (SAEs) significantly increase the risk of further SAEs and mortality. They performed a financial database analysis including patients treated with COPD from 1990 to 2005.9 However, since then general awareness of the disease has increased, and the number of available therapies for COPD has increased substantially. The past decades have marked the introduction of combined therapies of inhaled corticosteroids (ICS), long-acting beta agonists and muscarinic antagonists (LABA and LAMA) in dual and, recently, even triple combinations. Dual combinations have repeatedly been proven superior to single therapies for improving symptoms, lung function and quality of life and preventing AEs,1,10–12 and triple combinations are considered even more effective, with some studies even showing an increase in overall survival.1,13,14
In Hungary, COPD is the second most prevalent lung disease, with almost 200,000 registered patients.15 Although all novel therapies are available to patients, COPD is still considered an underdiagnosed and undertreated disease. Furthermore, there is a lack of data on exacerbation and mortality rates in Hungarian patients with COPD; thus, a comparison of our health system to international trends had not been possible so far. Finally, since the update of the GOLD guidelines in 2015, COPD treatment has been guided by symptoms and exacerbation risk. Two or more moderate exacerbations in one year increase the risk for further exacerbations, thus warranting treatment escalation. However, we do not currently know how moderate exacerbations fit into the model of successive SAEs described by Suissa et al.
Considering all these findings, we decided to implement and expand the methodology of Suissa et al. using the database of the Hungarian National Health Insurance Fund (NHIF), analysing the data on all COPD patients from 2009 to the end of 2019, incorporating the effect of moderate exacerbations into our model.
MethodsData sourceA longitudinal, retrospective analysis using the NHIF database was performed. This is a complex database that encompasses almost the entire population of Hungary, collecting certain healthcare data, including all reimbursement for drug prescriptions, inpatient and outpatient visits, laboratory and imaging examinations and the International Classification of Diseases (ICD-10) codes for all these events. All these data are linked to individual patients by their insurance number. The NHIF has a legal right to handle patients' data (Act No. 80/1997 on mandatory health insurance coverage) and has a right to share it on a claim basis (based on Act 63/2012 on the reuse of public data). Due to the contract terms, it is not possible to obtain data on individual patients or results that come from aggregating the data of less than 10 individuals from the database. Permission to conduct the study was provided by the National Institution of Pharmacy and Nutrition of Hungary, based on the beneficial assessment by the National Scientific and Research Ethics Committee of Hungary (docket number: IV/8716- 3 /2021/EKU).
This database had been used in previous studies in multiple fields of medicine (oncology,16,17 psychiatry,18,19 diabetology20 etc.); however, to the best of our knowledge, this is the first study in the field of obstructive pulmonary diseases.
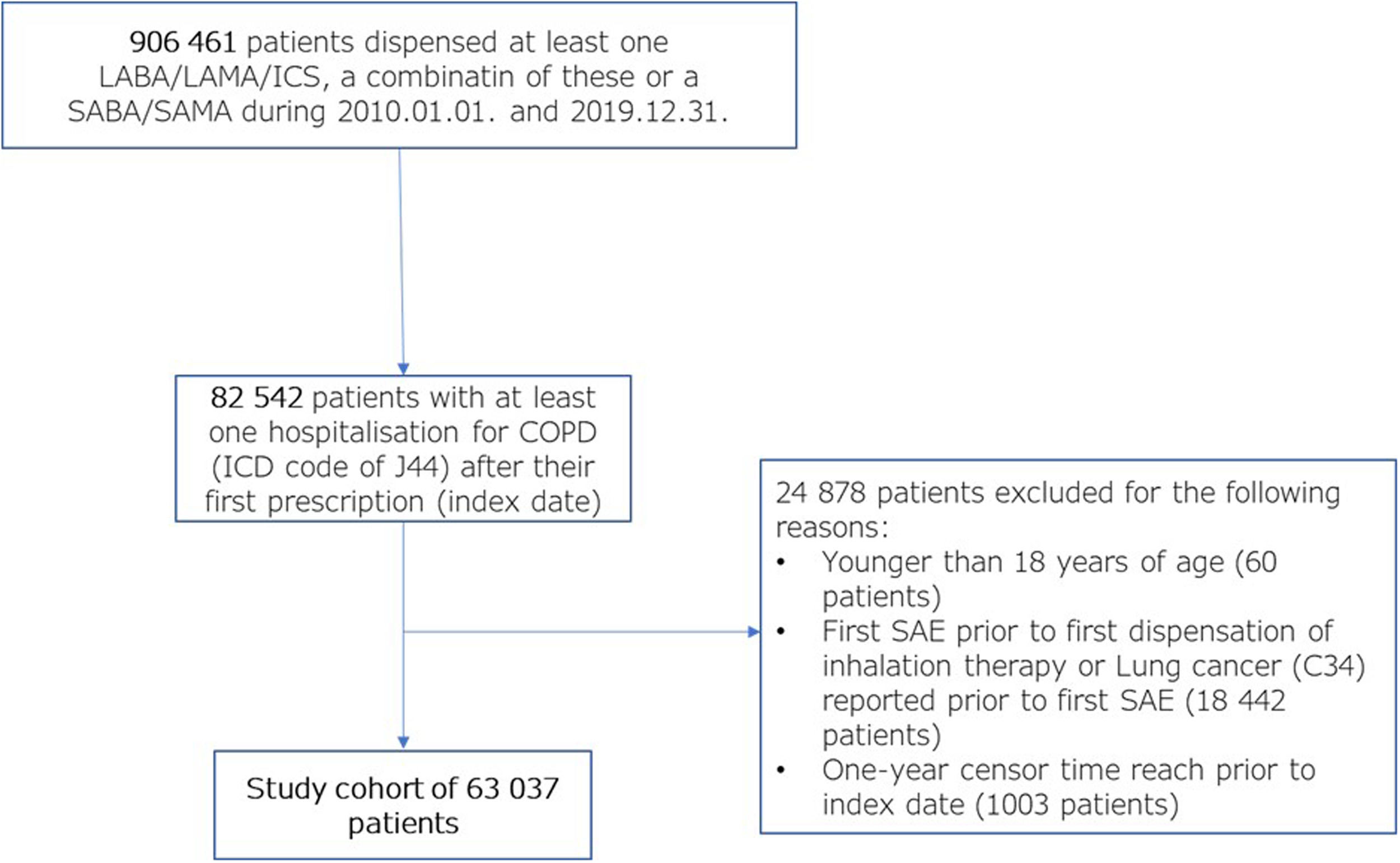
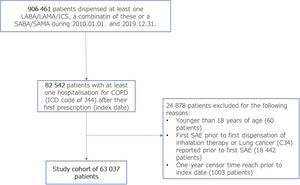
PatientsFirst, all patients who received at least one inhalation drug (ICS, LABA, LAMA or any fixed combination of these or SABA or SAMA) between 2010-01-01 and 2019-12-31 (906,461 patients) were identified. After the first respiratory medication, at least one hospital admission (inpatient hospital or ambulatory emergency care) with the J44 ICD-10 code was required (referred to as the index hospitalization event in the following sections) (82,542 patients – 100%). The baseline period was defined as one year before the index hospitalization event. Patients who were under 18 years of age (60 patients – 0.07%) or had been hospitalized with the J44 ICD-10 code one year prior to the first COPD drug dispensation or had lung cancer (C34 ICD-10 code) in the baseline period were excluded from the study (18,442 patients – 22.34%). Lung cancer can present with specific symptoms and can cause repeated hospitalizations in patients with COPD before its diagnosis; these events could be mistaken for SAE. Therefore, the follow-up of patients with lung cancer (code C34 ICD-10) was censored one year prior to the first discharge diagnosis of lung cancer. Therefore, patients who had lung cancer in the first year after the index hospitalization event were also excluded from the study, resulting in a final population of 63 037 (74.5%) patients. Follow-up of all patients continued until the end of the study (2019.12.31.), the date of death, or one year before the occurrence of lung cancer. During the follow-up time, all SAEs were recorded. The steps of cohort formation are shown in Fig. 1.
Flow chart of cohort formation.
COPD – Chronic Obstructive Pulmonary Disease; ICD-10 – 10th edition of International Classification of Diseases; LABA – Long-acting beta agonist; LAMA – Long-acting muscarinic antagonist; SABA – Short-acting beta agonist; SAE – Severe acute exacerbation; SAMA – Short-acting muscarinic antagonist.
Descriptive parameters such as gender, age (calculated at the first event of exacerbation), and length and type (acute inpatient care/rehabilitation) of the first hospitalization were collected on the index date. One year before the first severe exacerbation, a baseline period was applied, which was used to collect information on comorbidities and dispensed treatments. The Charlson comorbidity index was also calculated based on the definition of Quan.21
The main outcomes of the study were subsequent exacerbations and death. As in the method of Suissa et al., the start of follow-up differed for the two types of outcomes. In the case of a subsequent exacerbation, the start was defined as the date of live discharge from the hospital, while in the case of death, it was defined as the date of the index hospitalization event. The survival function of mortality was estimated using the Kaplan-Meier technique. The overall hazard function of successive severe exacerbations was estimated using the additional approach of Suissa et al., where the event was defined as the next severe COPD exacerbation or death, whichever occurred first, and with a competing risk model against death. To graphically analyse the effect of successive severe exacerbations, the hazard function of each successive severe exacerbation was combined in the same figure in such a way that two consecutive hazard functions were bound together when the previous one reached the median value of the survival probability function. Finally, competing risk models were used to estimate the effect of each successive severe exacerbation on the subsequent one and on mortality. The number of previous severe and moderate exacerbations was included in both models as a time-dependent covariate, maximized at a value of 10, with sex, age, and Charlson's comorbidity index as time-independent covariates. The values of the Charlson index were classified in the model as mild (values of 1–2), moderate (3–4) or severe (5), according to the appropriate methodology.22 In the case of the exacerbation model, the duration of the index hospitalization was also included as a covariate.9 Prescription of oral corticosteroids under J44 ICD-10 code (or 1430 diagnosis related group code DRG) OR prescription of any antibiotic under J44 ICD-10 were considered to be a moderate exacerbation. Subsequent prescriptions within 7 days were counted as the same moderate exacerbation event. This definition was similar to those used in previous trials.23,24 If an OCS prescription was followed by an SAE within 7 days, we only considered it as an SAE.
ResultsDescriptive resultsFollowing the application of all exclusion criteria, a final cohort of 63,037 patients was formed. In all, 32,227 patients were women (51.1%), and the average age was 67.4 years. Most of the patients had at least one comorbidity, with an average Charlson comorbidity index of 2.76. Most of the patients had received therapeutic regimens containing LABA (67.9%), ICS (54.9%) and LAMA (54.5%) one year before their index hospitalization. Almost two-thirds of all patients had used at least one reliever therapy (61.8%), and 37.0% had been administered more than three containers in the baseline year. Less than 1% of all patients received no therapy prior to their first SAE (0.7%). There were significant and clinically relevant differences between the general population, patients who suffered subsequent SAE and patients who died during follow-up. Members of the latter group were older and had more comorbidities (especially cardiovascular diseases), and a higher proportion of them were men. All other baseline details are shown in Table 1.
Characteristics of the entire patient population, patients who had suffered at least one subsequent SAE and patients who died during follow-up. For age, data on the Charlson index and sex are shown as mean and standard deviation. For all other variables, data are shown as the number of patients and percentages.
ICS – Inhaled corticosteroid; LABA – Long-acting beta agonist; LAMA – Long-acting muscarinic antagonist; SABA – Short-acting beta agonist; SAE – Severe acute exacerbation; SAMA – Short-acting muscarinic antagonist; SD – Standard deviation.
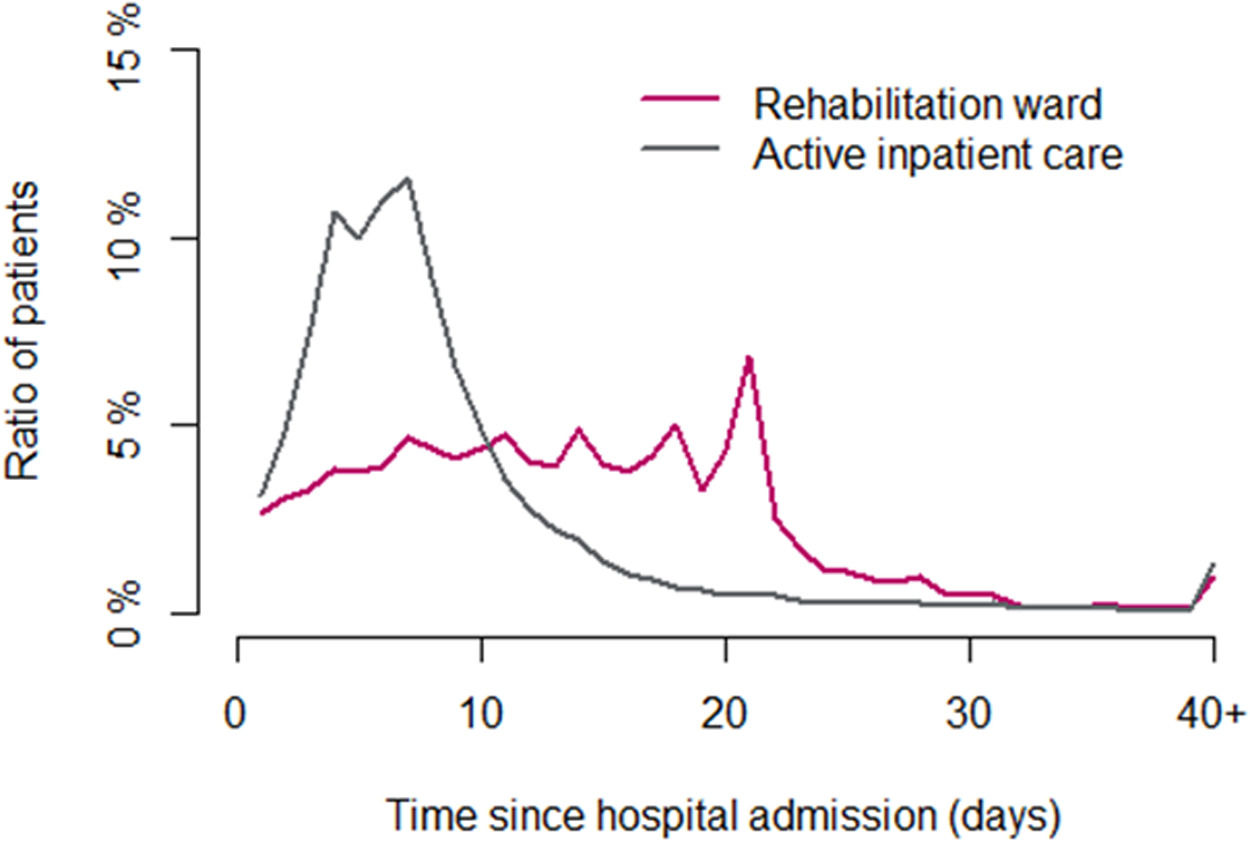
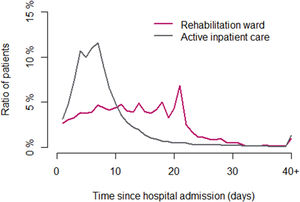
The mean follow-up was 1,209 days (3.31 years). At the index hospitalization, most patients (83.0%) had been treated in active inpatient care, with more than half of these patients (52%) spending 4–8 days hospitalized (average 8.51 days). However, about 17.0% of the patients had been treated in rehabilitation wards, where most of them spent 21 days. The distribution of patients according to the length of hospital stay (in days) is shown in Fig. 2.
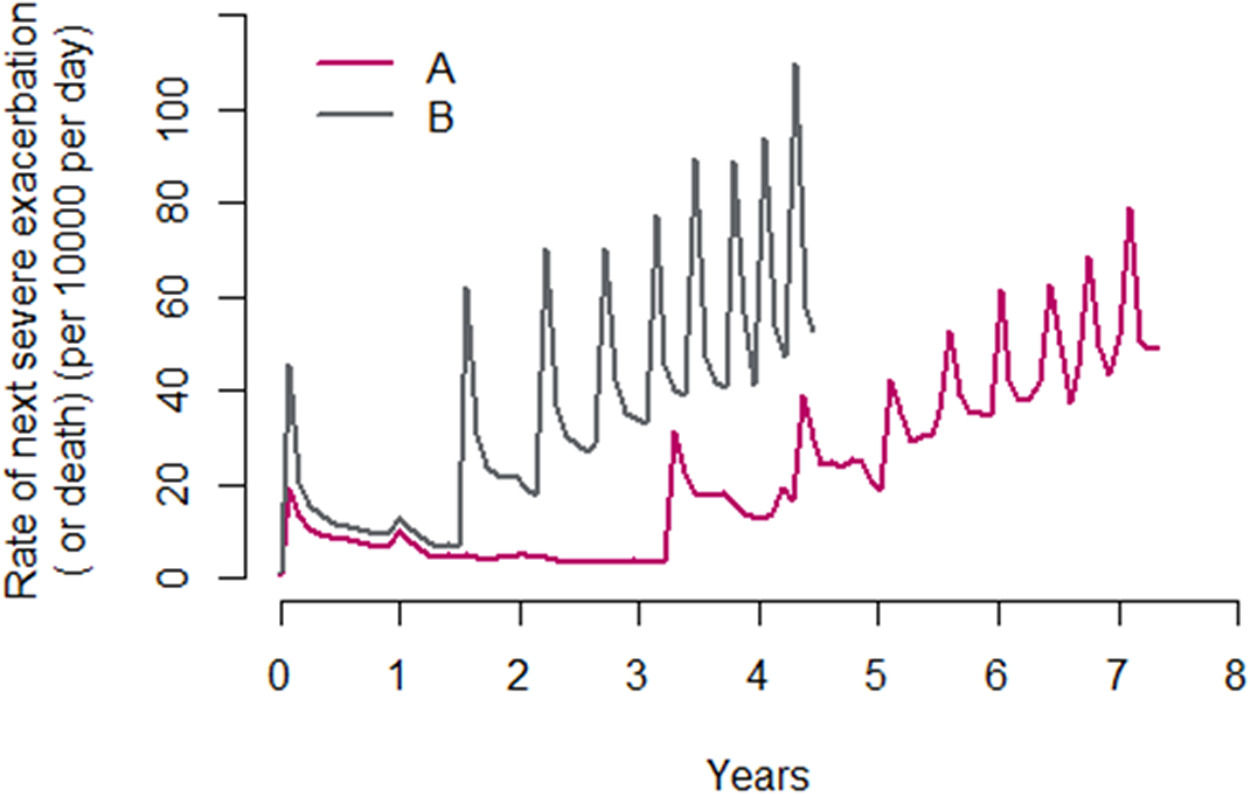
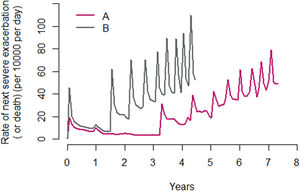
In all, 27,095 patients (43.0%) suffered at least one subsequent AE. As in Suissa et al., the median times between exacerbations decreased significantly with each subsequent SAE, and there was a parallel increase in the risk for the next SAE, from a median of 3.2 years between the first and second SAE to 0.3 years between the ninth and tenth SAE. The hazard function of all subsequent SAEs (A) and death (B), created by the same methods as Suissa et al, is shown on Fig. 3.
Hazard function of all subsequent SAEs (per 10,000 per day) from the time of the first SAE over the follow-up period, with the time between successive exacerbations estimated using (A) the median interexacerbation times, conditional on survival with death as a censor event, and (B) the median interexacerbation times as time to the next exacerbation or death, whichever occurred first.
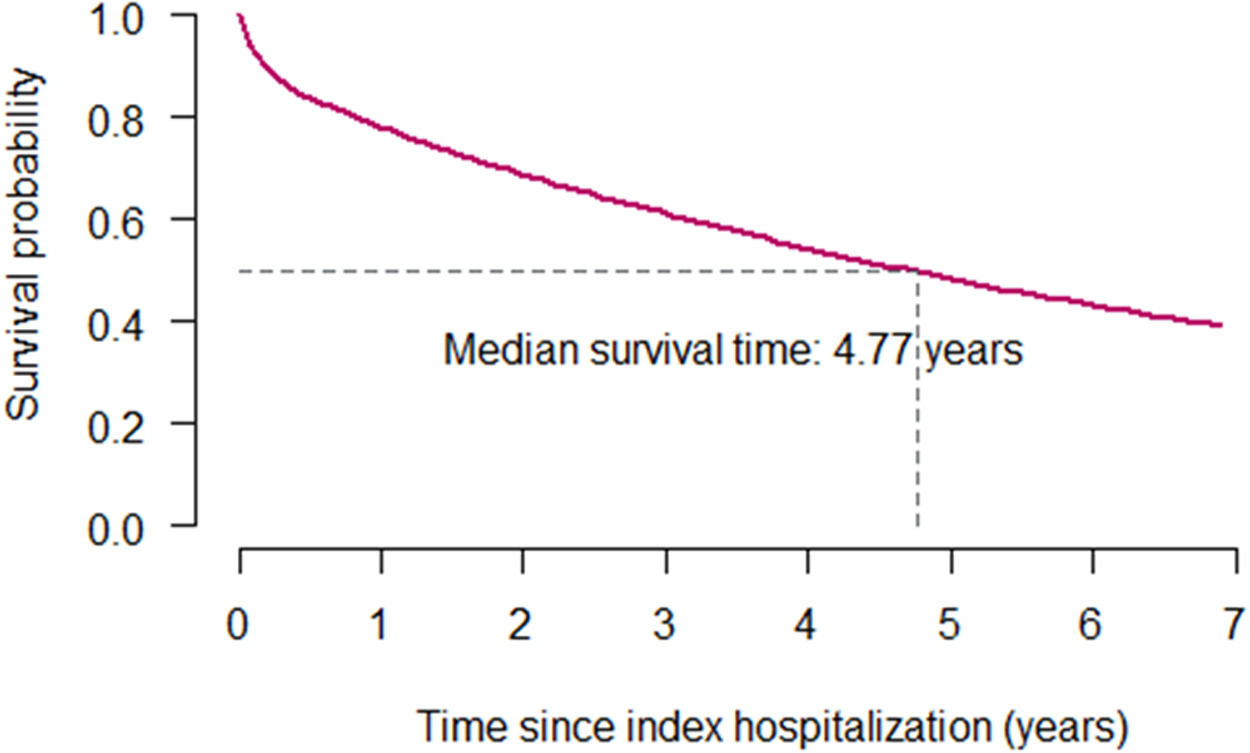
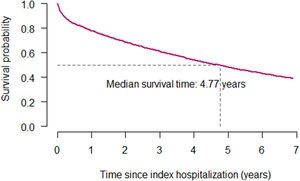
A total of 32,120 patients died during the follow-up period. The median survival was 4.77 years. The Kaplan-Meier survival curve for the whole cohort is shown in Fig. 4.
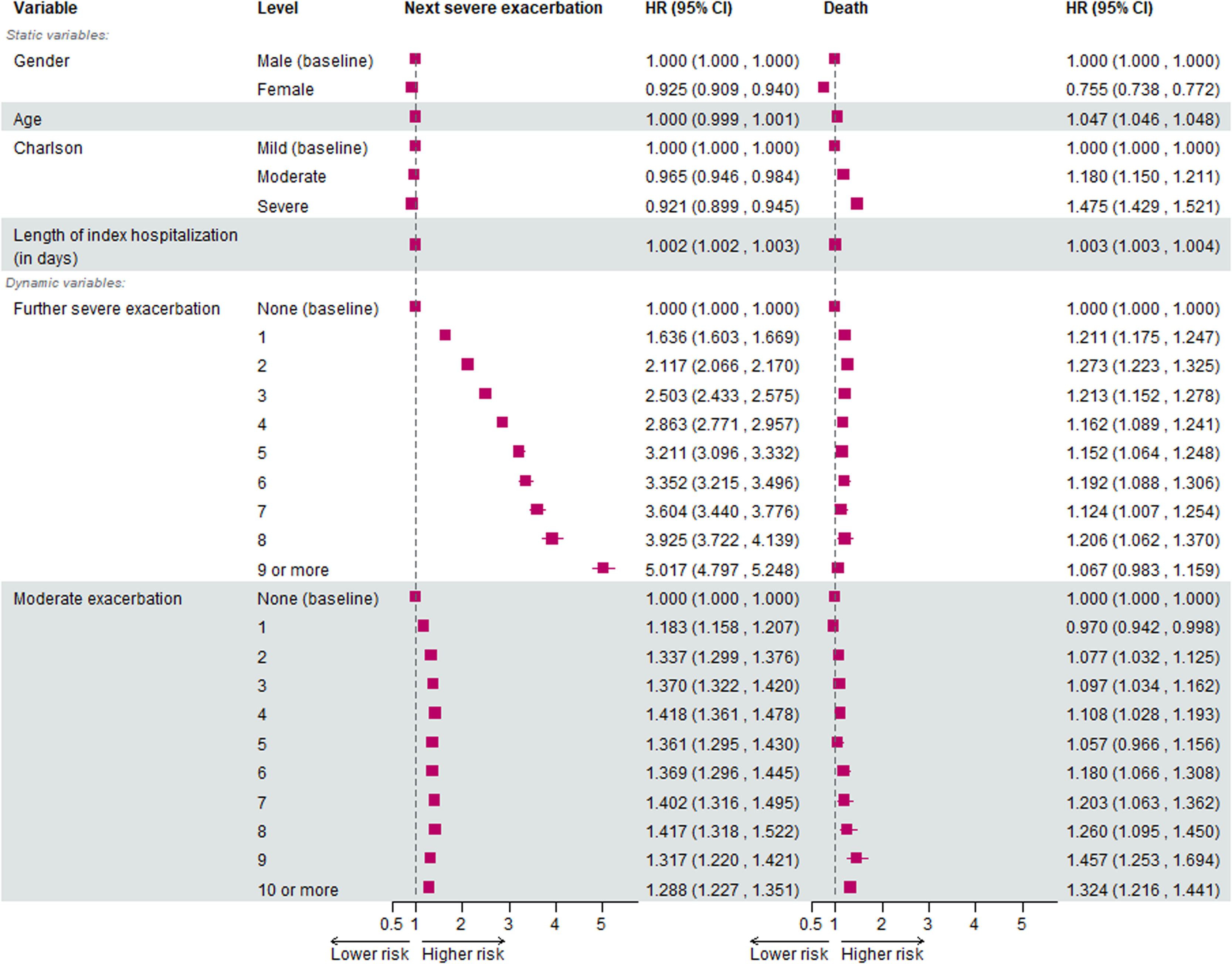
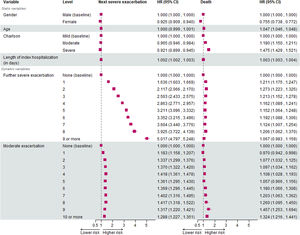
Model resultsIn the case of the exacerbation model, the risk of the next SAE increased from 1.636 (95% confidence interval – 95% CI: 1.602–1.669) to 5.017 (95% CI: 4.797–5.248) between the same SAEs. In the model, the effect of moderate exacerbations, the length of the index hospitalization and the Charlson comorbidity index on the risk for the next SAEs were also examined. Interestingly, moderate exacerbations increased the risk for the next exacerbation; however, this increase was not dependent on the number of moderate exacerbations (HR of 1.183 to 1.288 for the first and tenth or more moderate exacerbation). Female patients had a significantly lower risk of subsequent exacerbations (HR = 0.925). Although age did not have a significant effect, patients with a higher Charlson index score had a lower risk of subsequent SAEs. The longer the index hospitalization, the higher the risk of a subsequent SAE event. All HR values and 95% confidence intervals are shown in Fig. 5.
The trend in mortality risk after each subsequent SAE was different from that reported by Suissa et al. With the use of a competing risk model, the effect of SAEs on the risk of mortality did not increase with the number of the SAEs: HR = 1.211 (95% CI 1.175; 1.247) after the second SAE and 1.067 (0.983; 1.159) after nine or more SAE in the adjusted model. Moderate exacerbations increased the risk of mortality only after the second moderate exacerbation event (HR values of 1.077 and 1.324 for the second and tenth or more moderate exacerbations). Female patients had a markedly lower risk of death (HR = 0.755), while advancing age is associated with an increased risk of mortality. Finally, higher values of the Charlson index conferred a significant excess risk of death (HR = 1.180 and 1.475 for moderate and severe categories, respectively). All HR values and 95% confidence intervals are also shown in Fig. 5.
DiscussionIn this retrospective analysis based on the Hungarian NHIF financial database, we showed that each exacerbation requiring hospitalization (SAE) significantly increases the risk of the next SAE event and death. The result on SAEs, based on a 10-year follow-up of more than 63,000 COPD patients, is in line with the work of Suissa et al. from 2012, who first showed important and severe worsening of the disease after each SAE. In our analysis, the risk of mortality did not increase with the number of SAEs – a result of the competing risk model that was used. Patients are more likely to suffer a subsequent SAE after a previous one than they are to die. This results in a consistently elevated risk for mortality compared to baseline, without an increase after each subsequent event. When a competing risk model was not used, the risk for death increased with each subsequent SAE.
It is important to note that the increase in risk was far greater in their study (HR of 23.5 vs. 4.491 for the next SAE after the 10th exacerbation). Furthermore, the median survival was longer in our study (4.7 years vs. 3.6 years in Suissa et al.9); however, there was a difference in the average age of the included populations (67.4 years vs. 75.4 years9). Nonetheless, in our study, almost 50% of all patients had died within 5 years after their first SAE – an alarming number considering the high prevalence of COPD and its exacerbations. These results highlight the importance of exacerbation prevention and the need for further research seeking markers that could help physicians identify patients with an increased susceptibility to exacerbations. Moreover, the wider use of influenza and pneumococcal vaccination and non-pharmacological approaches such as pulmonary rehabilitation, physiotherapy and smoking cessation should be advocated for every COPD patient.
We could also demonstrate that moderate exacerbations increase the risk of a subsequent SAE and mortality. This is an important addition to earlier findings because it shows that moderate exacerbations are also pivotal events that could significantly alter the disease course. A more thorough assessment of all exacerbations is necessary for further improvement in their treatment – an idea that has been evoked more and more frequently in recent years.25
Moderate exacerbations can be treated with OCS and/or antibiotics in routine clinical care; however, in most financial database analyses, the prescription of only an oral corticosteroid is considered as a moderate AE. We believe that the main reason for this exclusion is that clinicians tend to prescribe antibiotics with ICD-10 codes other than J44, even if they aim to treat a moderate exacerbation. But the inclusion of ICD-10 codes other than J44 in the definition might mistakenly label other events (such as upper respiratory tract infections) as moderate exacerbations. For these reasons, we believe that the inclusion of an antibiotic prescription with a J44 code in the definition of moderate events will result in the inclusion of a higher number of moderate exacerbations, without the possibility of misidentification of events.
To explore the treatment prescription habits of the past decade, an analysis of therapies received after each SAE was also performed. Compared to baseline, there was an increase in the use of ICS-containing medications after the first two severe exacerbations (index hospitalization and first subsequent SAE). In all, 55% of all patients used ICS at baseline and 59% and 67% after index hospitalization and first subsequent SAE, respectively, but there was no further increase in the prevalence of ICS use after subsequent events. The GOLD guidelines have recommended ICS use after one severe or two (or more) moderate exacerbations for many years now.1 However, it was quite clear from our data that a high proportion of patients (almost one third) do not receive or do not take ICS-containing medications even after multiple SAE events. Without proper treatment, the prevention of further SAEs is impossible, as highlighted by a recent study by Tkacz et al., who showed that even a delay in appropriate treatment could also result in a large increase in the risk of exacerbation.25
The proportion of women among COPD patients has increased significantly in the past few decades. In our study, as in the study by Suissa et al., women also had a lower risk of subsequent SAEs and mortality. Based on our descriptive data, there were very few medically relevant differences between the enrolled women and men. While a higher proportion of men suffer from heart failure, prior myocardial infarction and peripheral vascular disease, many more women are reported to have depressive disorder or anxiety – both diseases could affect adherence to therapy, which could increase the risk of SAEs. In addition, more detailed research is needed to explain the reason for the lower risk of SAE among women.
The most important strength of our study is the large number of enrolled patients and a length of follow-up that could be difficult to match in prospective studies. Another advantage is that we could assess a population from the last decade, whose treatment had been much more uniform than that of patients in earlier studies. Finally, Hungary's health financing system is a single-payer system that includes almost the entire population of the country, resulting in a decrease in territorial or societal differences between the people included in the database and the entire population.
The most important limitation is the lack of data on the severity of COPD symptoms and lung function. Furthermore, it is impossible to verify the diagnosis of each patient based on spirometry data; inclusion was based solely on prescription medication use, and ICD-10 codes for prescriptions and discharge diagnoses. A further limitation is the lack of data on smoking history, obesity and possible exposure to high ambient air pollution.
Nonetheless, the results of our study and the conclusions drawn are of significance: despite the improvement in COPD management, the natural course of the disease cannot be altered, and exacerbations are still dominant effectors of the prognosis. Furthermore, these results highlight the huge importance of prevention of exacerbations and the need for further research on clinical parameters predicting exacerbations.
ConclusionsWe performed a retrospective financial database analysis of more than 63 000 patients followed over a 10-year period and concluded that moderate and severe exacerbations of COPD significantly increase the risk of further exacerbations and mortality. This effect increases with the number of events in the event of severe exacerbations. These results emphasize the importance of prevention of these events and the prompt initiation of appropriate treatment.
CRediT authorship contribution statementB. Sánta: Conceptualization, Visualization, Funding acquisition, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. G. Tomisa: Conceptualization, Visualization, Funding acquisition, Formal analysis, Methodology. A. Horváth: Conceptualization, Visualization, Funding acquisition, Formal analysis, Methodology. T. Balázs: Conceptualization, Visualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. L. Németh: Conceptualization, Visualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. G. Gálffy: Conceptualization, Visualization, Funding acquisition, Formal analysis, Methodology.