Among adults, sleep apnea is more common in highlanders than in lowlanders. We evaluated the sleep apnea prevalence in children living at high altitude compared to age-matched low-altitude controls.
MethodsHealthy children, 7-14 y of age, living at 2500-3800m in the Tien Shan mountains, Kyrgyzstan, were prospectively studied in a health post at 3250m. Healthy controls of similar age living at 700-800m were studied in a University Hospital at 760m in Bishkek. Assessments included respiratory sleep studies scored according to pediatric standards, clinical examination, medical history, and the pediatric sleep questionnaire (PSQ, range 0 to 1 with increasing symptoms).
ResultsIn children living at high altitude (n = 37, 17 girls, median [quartiles] age 10.8y [9.6;13.0]), sleep studies revealed: mean nocturnal pulse oximetry 90% (89;91), oxygen desaturation index (ODI, >3% dips in pulse oximetry) 4.3/h (2.5;6.7), apnea/hypopnea index (AHI) total 1.7/h (1.0;3.6), central 1.6/h (1.0;3.3), PSQ 0.27 (0.18;0.45). In low-altitude controls (n=41, 17 girls, age 11.6y [9.5;13.0], between-groups comparison of age P=0.69) sleep studies revealed: pulse oximetry 97% (96;97), ODI 0.7/h (0.2;1.2), AHI total 0.4/h (0.1;1.0), central 0.3/h (0.1;0.7), PSQ 0.18 (0.14;0.31); P<0.05, all corresponding between-group comparisons.
ConclusionsIn school-age children living at high altitude, nocturnal oxygen saturation was lower, and the total and central AHI were higher compared to children living at low altitude. The greater score of sleep symptoms in children residing at high altitude suggests a potential clinical relevance of the nocturnal hypoxemia and subtle sleep-related breathing disturbances.
World-wide, millions of people permanently reside at high altitudes above 2500 m and even more people travel to high altitudes for professional or leisure activities. Life-long exposure to hypoxia may have adverse health effects and cause chronic altitude illness such as chronic mountain sickness or high altitude pulmonary hypertension (HAPH), both associated with impaired quality of life and survival.1,2 In Kyrgyz highlanders living at altitudes above 2500 m, we observed a significantly higher prevalence of sleep apnea compared to lowlanders, in particular in those with HAPH.3 Highlanders with HAPH and sleep apnea performed worse in tests of vigilance and cognitive performance and their quality of life was reduced compared to healthy highlanders and lowlanders.
In children, health consequences of acute and chronic high-altitude exposure have not been extensively studied.4-8 Children permanently living at high altitude seem to have a reduced rate and pattern of growth9 but this may be modified by ethnicity.10 Based on a systematic review of the literature, oxygen saturation in children, 0 to 19 years of age, living at high altitude, decreases with higher altitude but shows a wide inter-individual variability and pronounced differences between sleep and wakefulness states.11 This variation regresses partially with advancing age from early childhood to adolescence. Both chronic and acute altitude exposure seems to affect cognitive performance among children and adolescents.12-14 From the scant published data it is not clear whether hypobaric hypoxia per se or other environmental influences, nutrition, socioeconomic conditions, genetics and further unknown factors are responsible for the health consequences in children living at high altitude described here.
In children living at low altitude, the prevalence of sleep disordered breathing (defined by an apnea/hypopnea index, AHI, ≥5/h) has been estimated at 1.2%, i.e., seems to be lower than that in adults if the same criteria (AHI ≥5/h) are applied.15 In children affected by obstructive sleep apnea, sleep disordered breathing may cause behavioral disturbances, cognitive impairment, retardation in growth, and, possibly, predispose to cardiovascular disease.16 Treatment may include adenotonsillectomy, continuous positive airway pressure (CPAP), weight management in obese children, nasal topical corticosteroids, among others.17-19
As the type and prevalence and the clinical manifestations of sleep disordered breathing in children living at high altitude have not been exhaustively studied, the purpose of this current investigation is to perform a cross-sectional survey including respiratory sleep studies and clinical evaluations in highlander school-age children in comparison to age-matched lowlander children. Based on our findings in adult highlanders,3 we hypothesize that children living at high altitude have a higher prevalence of sleep disordered breathing and nocturnal hypoxemia that negatively affects the health status of highland children.
MethodsDesign and settingThis is a cross-sectional study performed in children, 7-14 y of age, living in the Tien-Shan mountain range, near the Aksay health post, located at 3250 m, Kyrgyz Republic. Age-matched children living in the Bishkek area at 600-800 m were studied as controls in the National Center of Cardiology and Internal Medicine, Bishkek, at 760 m. The protocol was approved by the Ethics Committee of the National Centre of Cardiology and Internal Medicine. Written informed consent was obtained from children and parents.
ParticipantsTwo groups of healthy, male and female children, 7-14 y of age, were invited to participate in the study by word of mouth among families of presumed Kyrgyz origin known to the study staff and/or living in the area of the study locations: Group 1: Children living in the Aksay region at 2’500-3’800 m (high altitude group [HA-group]); group 2: Children living in the Bishkek area at 700-800 m (low altitude controls [LA-group]).
Exclusion criteria were any chronic or acute disease requiring medical treatment.
AssessmentsClinical examinationA general medical and sleep history was obtained and the pediatric sleep questionnaire (PSQ) was completed (see Supplementary Tables S1 and S2).20 The PSQ includes 22 items evaluating breathing, sleep, behaviour and some other aspects. The total PSQ score ranges from 0 to 1 with increasing prevalence of symptoms.
Chronic mountain sickness was assessed by the Qinghai score that includes 7 items evaluating breathlessness and/or palpitation, cyanosis, dilated liver veins, paresthesia, headache, tinnitus and sleep disturbance, each rated on a scale of no/mild/moderate/severe with 0-4 points. The chronic mountain sickness score is the sum of all answer scores plus 0-3 points for hemoglobin concentration.1 In the current study, only the sum of the answer scores (range 0-28) is reported. Acute mountain sickness (AMS) was evaluated by the Lake Louise questionnaire (LLS, 2018 version).21 It evaluates 4 symptoms (headache, gastrointestinal symptoms, fatigue and/or weakness, dizziness/light-headedness), each rated from 0 (absent) to 3 (severe). The sum of scores is the LLS with a range of 0-12 points.
A physical examination and pulse oximetry were performed. The presence of caries was recorded as the number of affected teeth and rated from 0 (absent) to 3 (3 or more affected teeth). Overbite and overjet (vertical overlap and horizontal distance, respectively, of upper vs. lower front teeth during occlusion) were measured. The Mallampati score of the pharyngeal space (range 1 to 4 with decreasing calibre) and the Brodsky score of tonsillar size (range 0 to 4 with increasing size) were obtained (Supplementary Table S3).22 Spirometry was performed (Easy One, NDD, Zurich Switzerland) using GLI reference equations.23
Respiratory sleep studiesRespiratory sleep studies were performed in a quiet room from 22:00 to 07:00 as described previously.6 One parent was allowed to stay with the child but instructed to avoid any disturbance. A portable polygraph (Alice PDX, Philips Respironics, Zofingen, Switzerland) recorded pulse oximetry (SpO2), respiratory inductance plethysmography of rib cage and abdomen, nasal cannula pressure swings, electrocardiogram and body position. Continuous audio-visual recordings were obtained by a low-light infrared camera.
Recordings were analyzed according to the American Academy of Sleep Medicine pediatric rules (see Supplementary Methods) from lights-off to lights-on (=time in bed, TIB).24,25 An obstructive apnea was defined as a ≥90% decrease in nasal pressure amplitude for the duration of ≥2 breaths with persistent respiratory effort as evidenced by chest wall excursions; a central apnea was scored in the absence of effort if the event lasted for ≥20 s or for ≥2 breaths in association with a SpO2 dip ≥3%. An obstructive hypopnea was scored if the nasal pressure swing amplitude dropped by ≥30% from pre-event baseline for ≥2 breaths in association with a ≥3% SpO2 dip and persistent efforts and/or flattening of the inspiratory nasal pressure contour; a central hypopnea was scored if efforts and signs of inspiratory flow limitation were absent. Individual large breaths, more than twice the baseline amplitude, were scored as sighs26 and divided into isolated sighs, not associated with any discernable other event, or sighs associated with an oxygen desaturation or an apnea/hypopnea event (Supplementary Fig. S1). The apnea/hypopnea index (AHI), oxygen desaturation index (ODI, ≥3% dips) and sigh index were computed as mean number of events per hour of TIB. Behavioural wakefulness and sleep periods were determined by inspection of audio-visual recordings and physiological signals. Estimated behavioural total sleep time (bTST) and sleep efficiency (bTST/TIB) were computed.
Outcomes and sample size estimationThe main outcomes were the AHI and ODI, further outcomes were other variables from sleep studies and clinical characteristics. The study was powered with 80% to detect a minimally important difference in AHI of 1/h, assuming a SD of 1.2/h, two-sided alpha of 0.05.
Data analysis and statisticsPer-protocol analysis was performed on all available data. Descriptive statistics are presented as counts and proportions, and medians (quartiles). Between-group comparisons were performed by Mann-Whitney tests and median differences with 95% confidence intervals (CI). Further analyses included multivariable linear regression models exploring associations among the AHI, sex, age, height and weight. A probability of <0.05 or 95% confidence intervals excluding zero were considered to indicate statistical significance.
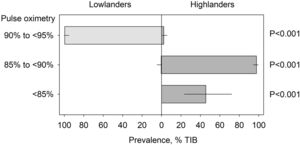
ResultsParticipantsAmong 60 children screened in Bishkek (LA-group), 41 were included; among 63 children screened in Aksay (HA-group), 47 were included (see participant flow in Supplementary Fig. S3). In the HA-group, 3 sleep studies were not available because of technical failure. Therefore, the per protocol HA-group consisted of 37 children (20 boys, 17 girls), the per-protocol LA-group of 41 children (24 boys, 17 girls). The sex distribution, age, height, weight and pulse rate in the two groups were similar (Table 1). However, the HA-group had a significantly lower median SpO2 of 92% (quartiles 91;94) and a higher respiratory rate of 23 breaths/min (21;24) than the LA-group (98% [97;99] and 20 breaths/min [19;22]). The assessment of the upper airways and teeth revealed similar, mostly normal findings in both groups. Spirometry showed significantly higher FVC and FEV1 in highlanders compared to lowlanders, FEV1/FVC was the same in both populations. The PSQ and the Qinghai chronic mountain sickness scores were significantly higher in the HA-group compared to the LA-group. Various aspects of the sleep history, including the median duration of the nocturnal rest period of 10.0 h (quartiles 9.0;10.5), did not significantly differ between groups although the nocturnal rest period of HA-group started and ended one hour later than those of the LA-group (Supplementary Table S4).
Participant characteristics.
Values are counts (percent) or medians (quartiles). The ranges indicated for several assessments reflects more severe symptoms or more abnormal findings with increasing numbers. For spirometry, data are available for 71 children (LA=36, HA=35) as some were unable to perform the maneuvers. FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second.
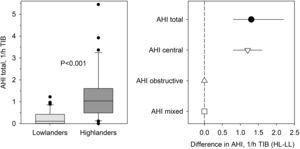
Results from sleep studies are summarized in Table 2 and illustrated in the Figs. 1 and 2. Both groups spent a similar period of approximately 9 h in bed. In the LA-group, the median SpO2 of 97% (quartiles 96;97), the median ODI of 0.7/h TIB (0.2;1.2) and the total AHI of 0.4/h TIB (0.1;1.0) were normal. In contrast, in the HA-group, the median SpO2 of 90% (89;91) and other indices of oxygenation were significantly reduced, while the total AHI of 1.7/h (1.0;3.6) and the ODI of 4.3 (2.5;6.7) were significantly elevated due to a higher number of central apneas/hypopneas (Table 2). Periodic breathing (sequences of at least 3 successive apnea/hypopnea cycles), was not observed in any of the groups. The sigh index was similar in both groups but the HA-group had less isolated and more associated sighs (followed by an apnea/hypopnea or desaturation) compared to the LA-group. The HA-group had a significantly higher heart rate than the LA-group (median difference of 8.1/min, 95% CI 4.1 to 21.1). The behavioral sleep efficiency (bTST) was reduced (86% [79;91]) and the sleep latency was prolonged (59 min [40;78]) in the HA-group compared to the LA-group, (92% [83;95] and 39 min [22;71]). Correspondingly, respiratory events referenced to estimated bTST differed slightly to those referenced to TIB but the trends of differences between groups remained unchanged (Supplementary Table S5).
Sleep studies in children living at high vs. low altitude.
Values are medians (quartiles) and median differences with 95% confidence intervals (CI) during time in bed (TIB). Heart rate is computed during behavioral total sleep time. SpO2: pulse oximetry; ODI: oxygen desaturation index, ≥3% dips; AHI: apnea/hypopnea index; %TIB: percent of time in bed; * the total sigh index includes the index of sighs without associated event (i.e., isolated sighs) and the index of sighs associated with an apnea/hypopnea or oxygen desaturation (i.e., associated sighs).
Nocturnal oxygenation in school-age children living at lowlands and highlands, respectively. The columns with lines represent medians and quartiles of the prevalence of pulse oximetry values in 3 different ranges in lowlanders (to the left) and highlanders (to the right). TIB = time in bed; HL-LL = values in highlanders minus corresponding values in lowlanders.
Apneas/hypopneas in school-age children living at lowlands and highlands, respectively. Left panel: the distribution of the total apnea/hypopnea index (AHI) in lowlanders and highlanders is represented by medians and quartiles (lines and boxes), 10th and 90th percentiles (whiskers) and values outside this range (dots). Right panel: median between-group differences with 95% confidence intervals in total, central, obstructive and mixed AHI.
To assess the effect of altitude on respiratory disturbance after adjusting for potential confounders, multiple regression models were fitted. They confirmed a significant effect of altitude on the total and central AHI even when controlled for age, sex, weight and height (Table 3). In further regression analyses exploring potential effects of altitude and total AHI on height, a negative association was found with altitude but not with the total AHI when controlled for age and sex and explored for the interaction of AHI and altitude (Supplementary Table S6).
Predictors of the apnea/hypopnea index in multivariable regression analysis.
R2 of the entire models for the total and central apnea/hypopnea index (AHI) were 0.40, P<0.001, both instances.
The current study in school-age children living at high altitude revealed lower indices of nocturnal oxygenation and a higher number of total and central apneas/hypopneas as well as a higher nocturnal heart rate compared to age-matched children living at low altitude. Since highlander children had higher questionnaire scores of sleep disturbance and chronic mountain sickness than their lowland counterparts, hypoxemia, respiratory sleep disturbances and other altitude-related factors may have affected the sleep quality and general well-being of highland children.
Our findings confirm the hypothesis that in children residence at high altitude is associated with hypoxemia and an elevated AHI compared to lowlander controls. The difference in AHI was due to more central events in highlander children which is consistent with our observations in unacclimatized prepubertal lowlander children travelling to 3450 m,6 and with studies in children, aged 3-5 years, residing at 1600 m (Denver, Colorado, USA).27 However, the findings seems to contrast to those of 2 studies performed in South America. Thus, in highlander children, 4-9 years of age, studied at 2560 m, near Bogota, the elevated AHI of 9.2/h was predominantly due to a higher frequency of obstructive events (8.8/h).28 Similarly, in children, 7-10 y and 13-16 y of age, studied at 3650 m in La Paz the obstructive AHI was higher (2.1/h) than the central AHI (0.7/h).29 Explanations of the apparent discrepancy in the prevalence of central and obstructive apneas/hypopneas among the current and the 2 South American studies may relate to differences in study procedures, and analysis of respiratory events28,29 as well as to differences in study populations including age, obesity in some children28 and, presumably, ethnicity. In accordance with previous studies in lowlander children acutely exposed to high altitude as well as in highlander children,6,29 sighs were commonly observed in the current study. The physiological significance of sighs is poorly understood, but they may be associated with arousals and interaction with respiratory control.26,30 Thus, sighs may trigger central apneas/hypopneas by transiently driving the arterial PCO2 below the apnea threshold, in particular, if the CO2 reserve is reduced due to an increased respiratory centre drive at altitude or in conditions such as in Down syndrome.31 In the current study, only a small fraction of sighs was associated with apneas/hypopneas but this type of sigh was more common in highlanders and may have contributed to their elevated central AHI.
Spirometry revealed greater dynamic lung volumes in highlander compared to lowlander children while FEV1/FVC ratios were similar (Table 1) which was consistent with observations in adult Kyrgyz highlanders vs. lowlanders.32 Whether the findings indicate greater pulmonary gas stores and, thus, a reduced plant gain of the respiratory control system in highlanders that dampens excessive overshooting of ventilation in response to the enhanced neural respiratory drive at high altitude requires further study.33
Highlander children had a higher heart rate than lowlanders suggesting sympathetic activation due to intermittent and sustained hypoxia as observed in healthy adults acutely exposed to high altitude,35 and in adult Kyrgyz highlanders with high altitude pulmonary hypertension (HAPH) who had also a greater incidence of cardiac arrhythmia and elevated markers of cardiovascular disease and cardiovascular mortality compared to lowlanders.3,34
The highlander children reported more sleep apnea-associated symptoms as evaluated by the PSQ, and their scores in the Qinghai chronic mountain sickness questionnaire were higher than those of lowlanders. Therefore, even the slight elevation of the AHI observed in the current study in association with intermittent and sustained hypoxia seems to have affected the perceived well-being of the highlander children during daytime, a novel and clinically important finding.
As observed in adult Kyrgyz highlanders, the body height of highland children tended to be reduced compared to lowlanders.3 In multivariable regression analysis adjusting for sex and age, the body height was (negatively) associated with altitude but not with the AHI suggesting that hypoxemia and other, unspecified factors, such as nutrition and socioeconomic conditions, rather than sleep disordered breathing may have contributed to the differences in height among highlander and lowlander children. This is of particular interest, as obstructive sleep apnea in children at lowlands is well known to be associated with reduced growth.18,19
A potential limitation to the interpretation of our data derives from the fact that factors associated with residence at high vs. low altitude, including socio-economic conditions, nutrition, physical activity, and a recruitment bias of our convenience samples, may have modified the results.
ConclusionsThe current study shows that in school-age children living at high altitude, nocturnal oxygen saturation is lower, and the total and central AHI are higher compared to children living at low altitude. Higher symptom scores of sleep disturbance and chronic mountain sickness in children residing at high compared to low altitude emphasize the potential clinical relevance of high altitude exposure and its physiological consequences in terms of hypoxemia and sleep-related breathing disturbances. Further studies in children are needed to scrutinize altitude-related adverse effects on neurocognitive and cardiovascular function and on other aspects of health at long term.
FundingThis research was funded by the Swiss-Kyrgyz High Altitude Medicine and Research Initiative, Zurich, Switzerland, and Bishkek, Kyrgyz Republic.
We appreciate the logistical support provided by Simone Sutter and the help with data acquisition provided by Gulzada Mirzalieva, Kamila Magdieva and Aijan Taalaibekova.