Surgical lung biopsy is a technique that presents a morbi-mortality rate of considerable importance. We analyze our experience with surgical lung biopsies for the diagnosis of diffuse lung disease and the effect produced on the indications for surgical biopsy in these pathologies after the publication of the consensus of the ATS (American Thoracic Society) and ERS (European Respiratory Society) for Idiopathic Pulmonary Fibrosis (IPF).
Patients and methodsWe performed a retrospective review of 171 patients operated between January 1997 and December 2011. We divided the series into 2 groups: group 1 (operated between 1997 and 2002) and group 2 (operated between 2003 and 2011). Suspected preoperative diagnosis, respiratory status, pathological postoperative diagnoses, percentage of thoracotomies, mean postoperative stay and perioperative morbidity and mortality were analyzed.
ResultsGroup 1 consisted of 99 patients and group two 72. The most frequent postoperative diagnoses were: usual interstitial pneumonia and extrinsic allergic alveolitis. There were ten (5.84%) deaths. Death was caused by progressive respiratory failure that was related to interstitial lung disease in 7 (70%) of 10 cases, alveolar haemorrhage in 2 (20%) and heart failure in 1 (10%).
ConclusionsSince the publication of the ATS and ERS consensus on the IPF, we have observed a noticeable decrease in the number of indications for surgical lung biopsy. This technique, though simple, has a considerable morbidity and mortality.
A biópsia pulmonar cirúrgica é uma técnica com uma morbimortalidade não negligenciável. Este trabalho resulta da experiência adquirida na realização de biópsias pulmonares cirúrgicas para o diagnóstico da doença pulmonar intersticial difusa, bem como pelo efeito provocado sobre as indicações da biópsia cirúrgica nesta entidade, após a publicação do consenso da ATS (American Thoracic Society) e da ERS (European Respiratory Society, para Fibrose Pulmonar Idiopática (FPI), em 2000 e 2002.
MétodosRevisão retrospectiva de 171 doentes intervencionados entre Janeiro de 1997 e Dezembro de 2011. A série de doentes foi dividida em dois grupos: o grupo 1 (operados entre 1997 e 2002) e o grupo 2 (operados entre 2003 e 2011). Os registos efectuados foram a suspeita diagnóstica pré-operatória, o estado respiratório, o diagnóstico patológico pós-operatório, a percentagem de toracotomias, a média de internamento hospitalar, além da morbilidade e mortalidade intra-hospitalares.
ResultadosGrupo 1 constituído por 99 doentes e o grupo 2 por 72. Os diagnósticos pós-operatórios mais frequentes foram a pneumonia intersticial usual e a alveolite alérgica extrínseca. Houve 10 mortes (5,84%). Em 7 (70%) dos 10 casos, a morte foi causada por progressão da insuficiência respiratória provocada pela doença subjacente, em 2 (20%) por hemorragia alveolar, e em um caso (10%) por insuficiência cardíaca.
ConclusõesDesde a publicação do consenso da ATS e da ERS na FPI, observou-se uma clara diminuição no número de indicações para a biópsia pulmonar cirúrgica. Esta técnica, apesar de simples, tem uma considerável morbilidade e mortalidade.
The classification of interstitial lung disease (ILD) includes a broad and heterogeneous group of pathologies under a common clinical–radiological context.1,2 Thoracic surgery plays an important role in this entity.3 Surgical lung biopsy performed with minithoracothomy or video-assisted thoracoscopic surgery (VATS) is indicated in all cases where a specific diagnosis of ILD has not been reached after less invasive examinations, but it is not a technique without serious complications.4,5
It is well known that VATS means a shorter operating time, less incidence of postoperative complications and reduced hospital stay in regard to thoracotomy.6 Whether it should be performed must be assessed in each individual case and will depend on the patient's clinical status.
In 2000, the ATS (American Thoracic Society) and the ERS (European Respiratory Society) published the first international consensus as regards the diagnosis and treatment of Idiopathic Pulmonary Fibrosis (IPF).7 Two years later, the classification of ILD was modified following a new consensus developed by the two societies.8 With this double consensus, the clinical and radiological criteria for the diagnosis of IPF were established without pathological confirmation. There are 4 major criteria and 4 minor criteria. Diagnosis of IPF is achieved in the presence of the 4 major criteria and at least 3 minor.
Here is our surgical experience in the diagnosis of ILD. We analyze the effect produced on the indications of the surgical lung biopsy in this group of entities after the publication of the consensus of ATS and ERS.
Patients and methodsWe conducted a retrospective study which included 171 patients operated in our department between 1997 and 2011 to obtain a lung biopsy in the ILD diagnosis process. These patients were classified in two groups: those operated between 1997 and 2002 (group 1) and those operated between 2003 and 2011 (group 2).
To divide our series we chose year 2003 as the turning point, this was one year after the publication of the last consensus of the ATS and ERS on ILD.7,8 We think that this is long enough to asses the influence of the two consensus on the indications of surgical lung biopsy.
We performed a right or left VATS by means 2 or 3 ports of 12mm. When it was impossible to get to a collapsed lung or when the patient did not tolerate clamping, an anterior thoracotomy was performed. Two or three specimens were obtained with endostaplers. The assessed variables in this study were: preoperative presumptive diagnosis, respiratory status, postoperative histological diagnosis, approach, mean postoperative hospital stay, postoperative morbidity, intraoperative and postoperative mortality and causes.
Statistical analysis was performed using Statistical Package for Social Sciences version 9.0 for Windows (SPSS, Chicago IL, USA). Age was expressed as mean and standard deviation (SD). All other categorical variables were summarized as counts and percentages. Results were considered to be significant if p was less than 0.05. We used the Chi-squared test and Fisher's exact test for qualitative variables. For quantitative variables we used the Mann–Whitney U test.
ResultsOf the 171 patients, 82 (48%) were men and 89 (52%) were women. The mean age was 57.29 years (SD 13.46). There were no significant differences with regard to age between the two sexes. Preoperative FEV1 (%) was 76.19±21.96, FVC (%) was 77.15±23.57 and DLCO (%) was 56.18±16.66.
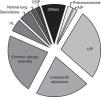
The preoperative presumptive diagnosis was: ILD without specifications in 142 patients (83.04%), sarcoidosis in 7 (4.09%), extrinsic allergic alveolitis in 7 (4.09%), Wegener disease in 3 (1.75%), Langerhans cell histiocytosis in 3 (1.75%), vasculitis in 3 (1.75%), drug-associated ILD in 2 (1.16%), rheumatoid lung in 1 (0.58%), lymphangioleiomyomatosis in 1 (0.58%), ILD and respiratory failure in 1 (0.58%) and Gaucher's disease in 1 (0.58%).
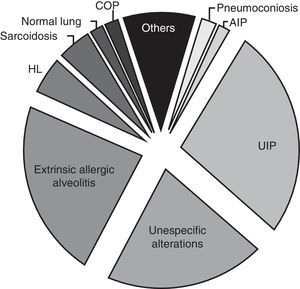
The postoperative histological diagnosis was: usual interstitial pneumonia (UIP) in 49 of the 171 cases (28.65%), extrinsic allergic alveolitis in 42 (24.56%), nonspecific alterations in 37 (21.63%), honeycombing lung in 9 (5.26%), sarcoidosis in 8 (4.67%), pneumoconiosis in 4 (2.33%), normal lung in 3 (1.75%), cryptogenic organizing pneumonia in 3 (1.75%), acute interstitial pneumonia in 3 (1.75%), granulomatous lymphomatosis in 3 (1.75%), carcinomatous lymphangitis in 2 (1.16%), Langerhans cell histiocytosis in 2 (1.16%), Wegener disease in 1 (0.64%), vasculitis in 1 (0.58%), deposit disease in 1 (0.58%), cytomegalovirus pneumonia in 1 (0.58%), bronchioalveolar carcinoma in 1 (0.58%), aspergillosis in 1 (0.58%). The diagnosis was made based on the light microscopy study. Immunohistochemistry techniques were useful in 12 of the 171 cases (Fig. 1).
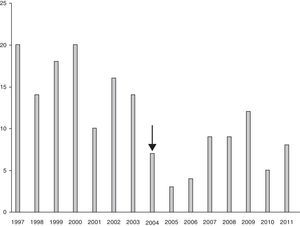
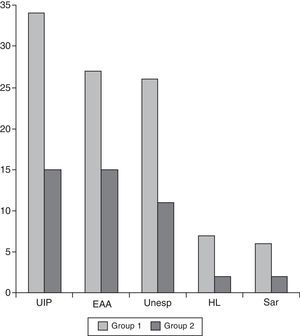
Comparing group 1 (operated between 1997 and 2002) and group 2 (operated between 2003 and 2011), we observed a decrease in the number of indications for surgical lung biopsies. In the first period 99 patients were operated, while in the second period 72 patients were operated. Moreover, we can observe that from year 2004 (two years after publication of the recommendations) there was a noticeable decrease in the number of biopsies performed when we compare the results with the previous years (Fig. 2). Also, when we compared the postoperative diagnosis between both groups, we observed a small decrease in the number of diagnoses of unspecific biopsy (28 in group 1 and 9 in group 2) and honeycombing lung (7 in group 1 and 2 in group 2) (Fig. 3).
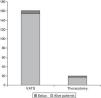
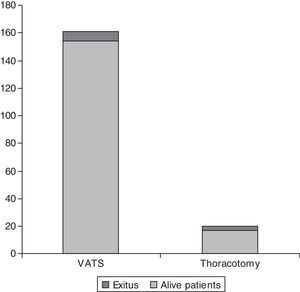
Of the 171 cases, 6 (3.50%) needed a minithoracotomy due to adhesions, 2 for lack of collapsed lung (1.16%) and 9 (5.26%) due to patient intolerance to one-lung ventilation. There were 10 deaths (5.84%) between 0 and 33 days after surgery (mean 7.67 days; SD 10.82 days). Differences in age and sex among these 10 patients and the rest of the series were not statistically significant. Causes of death were progression in respiratory failure due to underlying disease in 7 of the 10 cases (70%), alveolar haemorrhage in 2 of the 10 cases (20%) and heart failure in 1 of the 10 cases (10%). Intraoperative mortality was nil. Mortality was higher in the group of patients that underwent a thoracotomy (4 of 15 patients; 26.66%) compared with the group of VATS (6 deaths in 141 cases; 4.25%). The p was <0.005 (Fig. 4). Also, the death rate in group 1 was (7 deaths) compared to group 2 (3 deaths), although this was not statistically significant.
The mean postoperative stay in the whole series was 6.12 days (interquartile range 3–6 days). The mean stay for patients that underwent a thoracotomy was 12 days (SD 7.12) versus, 5.02 (SD, 4.33) for those operated by VATS (p<0.05).
DiscussionOur series presents demographic characteristics that do not substantially differ from those described by Xaubet et al. in 2004.9 In their study of 23 centres of the Spanish territory with 511 unselected cases for surgical indication, these authors observed an average age of 61 years old with a slight predominance of males (ratio male/female 1.2:1). Our results present an average age of 57.29 years old and a male/female ratio of 1.08:1.
We observed a marked decreased in the number of biopsies performed from 2004. We attribute this to the published clinical–radiological criteria for the diagnosis of IPF without histological confirmation. This fact indicated that recommendations still took some time before being used in clinical practise. However, we must admit that our series is surgical, and we cannot demonstrate whether there is an increase in patients diagnosed of IPF by clinical or radiological criteria.
Again, comparing our results with the series of Xaubet et al., there are important differences in the most common diagnoses: sarcoidosis represents 14.9% in this series, whereas in our study it only represents 4.67%.9 In the review of epidemiological studies by Demedts et al.,10 sarcoidosis and IPF appear as the most common ILD. This discrepancy could be explained by the increased probability of a diagnosis of sarcoidosis before reaching a surgical biopsy. The clinical, radiological and laboratory data provide a diagnosis that can be confirmed by a transbronchial biopsy with a high diagnostic profitability.11,12 These could be a source of bias in a surgical series. In this way, Santillan-Doherty et al. got a diagnosis of sarcoidosis in only 2 cases (3.71%) of the 60 lung biopsies performed for ILD in Mexico.4
By contrast, we found a high percentage of biopsies with the diagnosis of extrinsic allergic alveolitis (a centrolobular process caused by the exposure to organic allergens)13 in our study. We do not know the real causes of this finding. It could be related to the higher exposure to common agents in a rural population. Another possible explanation could be the greater propensity of the clinicians to request a histological confirmation of an ILD that is considered “curable” and this carries important medicolegal and sociolegal connotations.
Most of the authors consider IPF to be the most frequent ILD. This statement is consistent with the 28.65% of usual interstitial pneumonia (UIP) diagnosed in our series. Nevertheless, we cannot say that all these patients were diagnosed of IPF since the relationship between IPF and UIP is not one-one. In other words, the diagnosis of IPF requires the clinical exclusion of any other cause of UIP.2,7,8 This information was not available to us in the majority of the cases since 83.04% of the patients were referred to our department to perform a biopsy with the generic diagnosis of ILD, and without any other clinical suspicion. This means, from our point of view, an inappropriate practise possibly caused by overconfidence in the histological study that could explain why 37 of 171 cases (21.63%) received a nonspecific pathological diagnosis. Diagnosis of the majority of ILD is based on using light microscopy to evaluate the proportion, localization and timing of a common histological pattern, and it is not free of susceptibility.14 In a multicenter study published in 2001, researchers observed a 15% discrepancy in the diagnosis of UIP among expert pathologists.15 The pathologist's interpretation of the findings will be more precise the greater his knowledge of the patient's clinical condition.3,12,15 In this sense, it is mandatory to optimize the communication between the clinician, surgeon and pathologist in order to obtain the maximum benefit from the technique. Similarly, Sánchez-Varilla et al.16 found differences between the diagnoses reached by a group of specialist pathologists as opposed to others without specialization in 10 (30.3%) of 33 cases of ILD studied. Of these, 9 were (40.9%) in the group of 22 interstitial idiopathic pneumonias.
Another crucial factor for the diagnostic yield of a lung biopsy is the quality of the samples, which must be of sufficient size and taken in the appropriate time course and localization. 5.26% of the biopsies in our series were reported as honeycomb lung. This again means a non-specific diagnosis, as this is the final pathway of multiple ILD.
It is important to avoid an excessive delay of the biopsy during the course of the disease. Thus, we could reduce the number of inconclusive histologies. The biopsies obtained in advanced disease are less profitable and frequently lead to complications.8 In addition, when selecting optimal areas for the biopsy, areas of high honeycomb in the computed tomography should be avoided. Areas of ground glass are preferred.3 Finally, the optimal size to obtain a biopsy also depends on the clinical suspicion.12 This shows, once again, the importance of the collaboration between specialists.
Surgical lung biopsy can provide different types of information on the patient with ILD: to confirm a specific diagnosis or to exclude a suspicion; to justify experimental treatments or those with significant side effects; to establish a prognosis or analyze the response to a treatment; to evaluate the indication of lung transplantation or to resolve a medicolegal conflict… But in all cases, it is highly important to formulate the correct clinical question and never forget the great surgical risk.11
In conclusion, the new clinical–radiological criteria for the diagnosis of IPF published in 2000 and 2002, had induced a marked decrease in the number of biopsies performed from 2004 in our series. Surgical lung biopsy, although technically simple, has a non-negligible morbidity and mortality in patients that suffer ILD. The optimal selection of the cases to minimize the mortality and to optimize the diagnostic performance is essential.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.