Pulmonary embolism (PE) is a common disease with variable symptoms and high overall mortality. The clinical relevance of the extent of PE is still debatable, and the role of anticoagulation in patients with subsegmental involvement has been contested. Our objective is to describe the clinical details of patients with PE in our hospital and to analyze their prognosis based on the extent of the disease.
Materials and methodsRetrospective study of 313 patients diagnosed with PE by chest computed tomography (CT) scan at the Hospital Complex of Pontevedra in Spain for six years. Predictors of mortality were determined by multivariate analysis.
ResultsWomen accounted for 56% of patients, and patient median age was 70 years (interquartile range 53–78 years). Subsegmental PE accounted for 7% of all cases; these patients were younger and had lower comorbidity; they reported chest pain more often, performed better in blood gas analysis and none of them had proximal deep vein thrombosis (DVT). Patients with subsegmental PE had a higher survival rate. Factors independently associated with mortality were cancer diagnosis and higher comorbidity.
ConclusionsPatients with subsegmental PE clinically differ from those with more proximal PE. Underlying diseases have more influence on the prognosis than the extent of the disease.
A embolia pulmonar (PE) é uma doença comum com sintomas variáveis e uma elevada taxa de mortalidade global. A relevância clínica da extensão da PE é ainda fonte de debate, e o papel da anticoagulação em pacientes com envolvimento de sub-segmentos foi contestado. O nosso objectivo é descrever os dados clínicos de doentes com PE no nosso hospital e analisar o seu prognóstico, com base na extensão da doença.
Materiais e métodosEstudo retrospectivo de 313 doentes, diagnosticados com PE, através de uma tomografia computadorizada de tórax (TC) no Complexo Hospitalar de Pontevedra, em Espanha, durante seis anos. Os preditores de mortalidade foram determinados por análise multivariada.
ResultadosAs mulheres representaram 56% dos doentes, e a idade média era de 70 anos (amplitude interquartis 53–78 anos). A PE ao nível dos sub-segmentos era responsável por 7% de todos os casos; estes doentes eram mais jovens e tinham uma taxa de comorbidade inferior; referiram dor torácica com maior frequência, tiveram melhores resultados na gasimetria arterial e nenhum deles sofreu uma trombose venosa profunda (TVP) proximal. Os doentes com PE ao nível dos sub-segmentos tiveram uma taxa de sobrevivência mais elevada. Os factores associados de modo independente com a mortalidade foram o diagnóstico de cancro e uma comorbidade mais elevada.
ConclusõesOs doentes com PE ao nível dos sub-segmentos diferem clinicamente daqueles com PE proximal. No prognóstico, as doenças subjacentes têm maior influência que a extensão da doença.
Pulmonary embolism (PE) is a common disease with an annual incidence of 110 cases per 100,000 adults,1 which has been increasing over recent years.1,2 Symptoms may vary, from asymptomatic to obstructive shock following circulatory collapse.3 Overall mortality is not only high, but also highly variable. In a recent study, overall mortality was 8.65% at three months.4 Central arteries are most commonly involved,5,6 while subsegmental PE accounts for 4–7% cases.7–9 However, a number of studies report subsegmental involvement in up to 30% patients.9
The clinical relevance of the extent of PE is still debatable, and the role of anticoagulation in patients with subsegmental involvement has been contested in a number of studies.10,11
The objective of our study was to describe the clinical particulars of patients with PE in our hospital and to analyze their prognosis based on the extent of the disease.
MethodsWe conducted a retrospective study of all patients above 18 years of age with confirmed CT chest scan diagnosis of PE12 at the Hospital Complex of Pontevedra in Spain between January 2005 and December 2010. Follow-up ended on January 31, 2012, the survival deadline. Thus, the minimum follow-up was 14 months. Administrative data from coded hospital discharges were used for patient selection. Those patients with incomplete data and/or diagnosis made by methods other than chest CT scan were excluded from the initial 470 patient sample. Thus, 313 cases were included in the study.
The following data were analyzed:
- 1.
Personal details: age, gender, active smoking and obesity (body mass index >30).
- 2.
Clinical, physical examination and additional test data: recent surgery, anesthesia, trauma, cancer, prolonged travel and/or immobilization; lower limb paralysis; insertion of central catheter, thrombophilia or antiphospholipid syndrome; previous venous thromboembolic disease (VTE); lower-limb varicose veins; pregnancy, contraception or hormone therapy. Tachypnea, crepitant rale, signs of lower-limb DVT; vital signs, systolic hypotension (SBP<100mmHg), diastolic hypotension (DBP<60mmHg) and tachycardia (heart rate>100).13,14 Arterial blood gas analysis, hemoglobin, hematocrit, platelet count, leukocytosis, neutrophilia, glucose, urea, creatinine, sodium, potassium and fibrinogen, stratified as normal or abnormal as per the laboratory reference values in our hospital. D-dimer levels were determined with high-sensitivity turbidimetric immunoassay. D-dimer levels above 500ng/ml were considered pathological. Renal function was considered normal with glomerular filtration rate as per the abbreviated MDRD equation above 60ml/min/1.73m2.15 Electrocardiographic and imaging findings: plain chest radiograph; lower-limb echo-Doppler (complete or distal) was performed at the discretion of the radiologist, based on patient clinical presentation. PEs were collected and classified into three groups with chest CT scanning: central (main trunk, right or left pulmonary artery, intermediate arteries or lobar arteries), segmental and subsegmental. Thrombus location was determined according to the largest artery involved. Comorbidity measured by the Charlson index (CCI), categorized into “0”, “1–2” and “≥3”.16
- 3.
Clinical probability was determined with the Wells17 and Geneva18 scores.
- 4.
Cause of death was attributed by the main investigator based on available medical records and categorized into three groups: PE-related, PE-unrelated, and unknown. Discordance between data and uncertain final diagnoses was resolved by discussion with data collectors.
Categorical variable data are presented as frequencies (percents); continuous variable data are presented as medians and interquartile ranges.
Fisher's exact and Chi-squared tests were used to compare categorical variables. Between groups, continuous variables were compared by ANOVA or the Kruskal–Wallis test with non-normal distributions.
Survival curves and increases in probability of death were respectively calculated with the Kaplan–Meier test and the Cox regression. Differences were considered significant at p<0.05. Data were analyzed with SPSS 15 for Windows.
ResultsThe study included 313 PE patients; 56% were women and the median age was 70 years (interquartile range 53–78 years). Central PE accounted for 68% of cases; segmental and subsegmental PE, for 25% and 7%. Patients with subsegmental PE were younger, had lower comorbidity and none of them presented proximal DVT (Table 1). Patients in the subsegmental PE group included 10 (45%) with single PE and 12 (55%) with multiple PE.
Clinical data and demographics (n=313).
| Total (n=313) | Central (n=213) | Segmental (n=78) | Subsegmental (n=22) | p | |
| Age | 70(53–78) | 71(56–79) | 70.5(51.5–78) | 55(38.35–71.5) | * |
| Gender(male) | 137(43.8%) | 89(42%) | 38(49%) | 10(45.5%) | NS |
| Symptoms | |||||
| Dyspnea | 181(57.8%) | 148(69.5%) | 25(32%) | 8(36.4%) | * |
| Orthopnea | 3(0.95%) | 3(1.4%) | 0(0%) | 0(0%) | NS |
| Chest pain | 132(42.2%) | 80(37.6%) | 40(51.3%) | 12(54.5%) | * |
| Pleuritic pain | 136(43.4%) | 85(39.9%) | 36(46.5%) | 15(68.2%) | * |
| Anginal pain | 28(8.9%) | 23(10.8%) | 4(5.1%) | 1(4.5%) | NS |
| L-limb edema/pain | 75(23.9%) | 54(25.4%) | 19(24.4%) | 2(9%) | NS |
| Hemoptysis | 15(4.8%) | 7(3.3%) | 6(7.7%) | 2(9%) | NS |
| Cough | 21(6.7%) | 13(6%) | 6(7.7%) | 2(9%) | NS |
| Palpitations | 18(5.75%) | 15(7%) | 2(2.6%) | 1(4.5%) | NS |
| Syncope | 38(12.1%) | 31(14.6%) | 4(5.1%) | 3(13.6%) | NS |
| Proximal DVT | 66(21%) | 52(24.4%) | 14(17.9%) | 0(0%) | ** |
| Charlson | ** | ||||
| =0 | 68(21.7%) | 37(17.4%) | 23(29.5%) | 8(36.4%) | |
| 1–2 | 135(43.1%) | 101(47.4%) | 23(29.5%) | 11(50%) | |
| ≥3 | 110(35.1%) | 75(35.2%) | 32(41%) | 3(13.6%) | |
| Cancer | 68(21.7%) | 47(69.1%) | 19(27.9%) | 2(2.9%) | NS |
| Probable PE (Wells) | 170(54.3%) | 110(51.6%) | 43(55%) | 17(77.3%) | NS |
| Probable PE (Geneva) | 216(69%) | 143(67%) | 54(62.23%) | 19(86.4%) | NS |
NS=non-significant.
Age as median and interquartile range.
As for symptoms, dyspnea is the most common one in patients with central PE, and also pain is common in segmental and subsegmental PE (Table 1). Comorbidity measured by CCI was present in 78% of cases, and was more frequent in central PE (Table 1). 22% of patients had been diagnosed with cancer prior to PE (data not shown). As for the presence of risk factors and the clinical probability of PE according to the Wells and Geneva scores, no differences were found on the extent of the disease (Table 1). As for the additional tests, patients with central artery involvement showed significantly higher D-dimer values and performed poorer in blood gas analysis. No significant difference was found in any of the remaining parameters (Table 2). All patients were admitted to the hospital and treated with anticoagulants.
Additional tests.
| Central (n=213) | Segmental (n=78) | Subsegmental | p (n=22) | |
| Hemoglobin | 13(11.7–14.1) | 13.2(11.7–14.3) | 13.4(12.3–14.5) | NS |
| Htc | 39(35–40) | 39(35.5–42.5) | 40.5(37.7–42.7) | NS |
| Leukocytes | 9400(7500–12,350) | 9095(6675–12,100) | 8650(6825–10,750) | NS |
| Neutrophils (%) | 75(62.5–81) | 75(66–83) | 79(62–84) | NS |
| Platelets | 232,000(184,750–285,500) | 252,000(189,000–320,000) | 238,500(190,750–267,250) | NS |
| Fibrinogen | 535(430–689) | 548(434–694) | 465(395–590) | NS |
| D-dimer | 3633(1977.5–5322.5, n=62) | 2230.7(1063–3124, n=23) | 1494(1104–1583, n=6) | * |
| Glucose | 108(94–140) | 103(90–129) | 105(94–138) | NS |
| Normal renal function | 149(71%) | 59(75.6%) | 17(77.3%) | NS |
| Sodium | 139(136–141) | 133(136–139) | 139(137–141) | NS |
| Potassium | 4.3(4–4.6) | 4.4(4.1–4.6) | 4.5(4.2–4.7) | NS |
| SatO2 (21%) | 92.7(88–94.7) | 93.2(89.2–95.2) | 95.4(94.1–96.4) | * |
| pH | 7.5(7.4–7.5) | 7.5(7.4–7.5) | 7.4(7.4–7.5) | NS |
| PO2 | 61(51–69) | 65(53–72) | 73(57–78) | * |
| PCO2 | 34(30–38) | 36(32–40) | 35(32–40) | * |
| Bicarbonate | 23(21–25.6) | 24.9(22.6–26.4) | 24(22.3–26.8) | ** |
| Normal chest X-ray | 116(54.4%) | 39(50%) | 13(59%) | NS |
NS=non-significant.
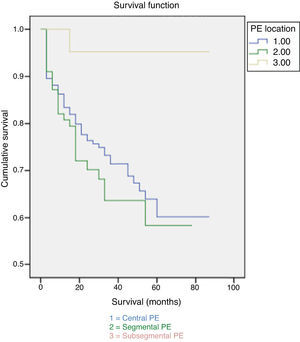
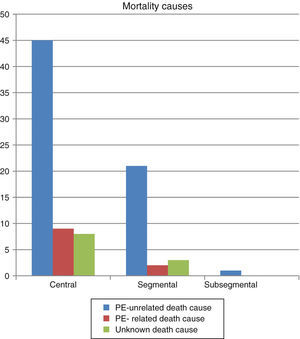
30-day mortality was 7%, attributable to PE in 3.5% of cases. Patients with subsegmental PE showed significantly higher survival rates both at 30 days and at the end of the follow-up (Fig. 1). No difference was observed in the cause of death based on the extent of PE (Fig. 2). Factors independently related to a higher likelihood of mortality were cancer diagnosis and higher comorbidity measured by the Charlson index (Table 3).
DiscussionPrevalence of subsegmental PE was 7%, similar to that reported by other authors.5 No significant gender difference was found in terms of the extent of the disease; however, the proportion of women is slightly higher in our sample. The age of patients with subsegmental PE was lower than that of patients with central and segmental PE. The difference could be related to hypercoagulation and the changes in vascular endothelium that come with aging19: these could facilitate the extent of thrombi in older patients, especially considering that no age difference was found in the prevalence of risk factors for PE.
Although symptoms of PE are nonspecific and presentation is usually similar in adults, we found chest pain in younger patients and a positive association between age and dyspnea.19 In our study, chest pain in younger patients with peripheral PE and dyspnea in older patients with central PE and higher levels of hypoxia were most often reported. In general, dyspnea occurred less frequently in patients with subsegmental PE.10 These performed better in blood gas analysis and had an overall less severe clinical presentation,20 including lower proximal DVT.5,10
D-dimer levels in patients with PE seems to be associated with thrombus size, are related to PE severity and have value as prognostic marker.21 We found lower serum levels in patients with subsegmental PE, who had a more benign clinical presentation and a better mid-term outcome. However, D-dimer testing was only carried out in 30% of cases. Thus, our results are not conclusive.
In our study, 30-day mortality was higher than in other studies,22 although the proportion of PE-related deaths was similar.4 None of the patients died as a result of subsegmental PE. Differences in mortality could be due to different causes: the mean age of our patients is higher, many have accompanying chronic diseases that can condition the bad outcome of PE, and the number of deaths from comorbidity is also high. In fact, the most important determining factor of mortality is comorbidity, especially cancer. Patients with cancer and PE not only have a bad prognosis, but also a worse one than patients with only cancer or PE.23 Symptoms of PE could be mistaken for those in exacerbations of previous cardiopulmonary diseases,24 leading to delayed diagnosis and treatment, worse prognosis and higher mortality. Older patients are more complex, and may have higher comorbidity and delayed diagnosis24; comorbidity has also been suggested as a risk factor for PE in these patients.25,26
The clinical significance of subsegmental PE is unknown. However, it may have clinical relevance and long-term consequences in patients with low cardiopulmonary reserve.2 It usually has low mortality2,27 and a good prognosis.5,28 Thus, a number of authors have suggested that certain patients would not require anticoagulant therapy.29,30
Our data suggest that patients with subsegmental PE clinically differ to a large degree from those with more proximal PE. The prognosis is influenced by the presence of accompanying disorders rather than by the extent of the disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
S. García Sanz for the translation of the manuscript.