Although the sweat test (ST) is considered the gold standard for the diagnosis of cystic fibrosis (CF), it still remains the center of great interest as well as debate over its execution and interpretation.1
We read carefully the important article by Traeger and colleagues.2 The study showed that in 13,775 ST [313 (2.3%) patients with CF], sweat chloride concentrations decrease in the first year of life, increase in the second year until the age of 18, and decrease slowly after age 18.
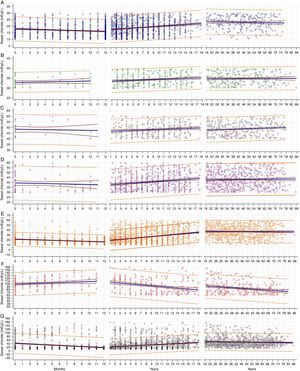
We assessed 5196 ST [671 (12.91%) patients with CF] in our university referral center. Unlike the study conducted by Traeger and colleagues, we assessed chloride levels by age, considering the reference values for the ST3,4 (Fig. 1).
Correlation between levels of sweat chloride and age of subjects undertaking sweat tests, considering possibilities of groups by chloride concentrations. (A) Chloride<30mEq/L: (age: ≤12 months) N=1178; Spearman's coefficient rank correlation (rho)=−0.159, 95%CI=−0.214 to −0.103; p<0.001. Coefficient of determination R2=0.025; y=17.9905+(−)0.1643x; p<0.001. (Age: >1 year to ≤18 years) N=1827; rho=0.192, 95%CI=0.148–0.236; p<0.001. R2=0.039; y=17.1432+0.2749x; p<0.001. (Age: >18 years) N=230; rho=−0.048, 95%CI=−0.176 to 0.082; p=0.469. R2=0.002; y=23.7087+(−)0.01622x; p=0.538. (B) Chloride≥30mEq/L to <40mEq/L: (age: ≤12 months) N=52; rho=0.097, 95%CI=−0.181 to 0.360; p=0.495. R2=0.013; y=32.8243+0.07205x; p=0.427. (Age: >1 year to ≤18 years) N=340; rho=0.151, 95%CI=0.045–0.253; p=0.005. R2=0.021; y=33.4949+0.09905x; p=0.007. (Age: >18 years) N=244; rho=0.026, 95%CI=−0.100 to 0.151; p=0.692. R2<0.001; y=34.6330+0.003532x; p=0.758. (C) Chloride≥40mEq/L to <60mEq/L: (Age: ≤12 months) N=33; rho=0.025, 95%CI=−0.321 to 0.365; p=0.892. R2<0.001; y=48.4653+(−)0.09778x; p=0.748. (Age: >1 year to ≤18 years) N=278; rho=0.134, 95%CI=0.016–0.248; p=0.026. R2=0.018; y=46.4328+0.1699x; p=0.026. (Age: >18 years) N=343; rho=0.117, 95%CI=0.011–0.220; p=0.031. R2=0.014; y=46.9588+0.03910x; p=0.031. (D) Chloride≥30mEq/L to <60mEq/L: (Age: ≤12 months) N=85; rho=−0.049, 95%CI=−0.259 to 0.166; p=0.656. R2=0.005; y=39.4291+(−)0.1577x; p=0.5224. (Age: >1 year to ≤18 years) N=618; rho=0.210, 95%CI=0.134–0.285; p<0.001. R2=0.042; y=37.2303+0.3772x; p<0.001. (Age: >18 years) N=587; rho=0.017, 95%CI=−0.064 to 0.098; p=0.678. R2<0.001; y=42.3785+0.01182x; p=0.565. (E) Chloride<60mEq/L: (Age: ≤12 months) N=1263; rho=−0.246, 95%CI=−0.297 to −0.193; p<0.001. R2=0.078; y=21.5579+(−)0.4503x; p<0.001. (Age: >1 year to ≤18 years) N=2445; rho=0.336, 95%CI=0.300–0.370; p<0.001. R2=0.127; y=17.9823+0.968.9x; p<0.001. (Age: >18 years) N=817; rho=−0.01, 95%CI=−0.078 to 0.0586; p=0.775. R2<0.001; y=37.6153+(−)0.007263x; p=0.776. (F) Chloride≥60mEq/L: (Age: ≤12 months) N=179; rho=0.134, 95%CI=−0.013 to 0.275; p=0.073. R2=0.021; y=99.9960+0.8686x; p=0.058. (Age: >1 year to ≤18 years) N=238; rho=−0.287, 95%CI=−0.400 to −0.166; p<0.001. R2=0.085; y=107.6871+(−)1.6094x; p<0.001. (Age: >18 years) N=254; rho=−0.190, 95%CI=−0.306 to −0.068; p=0.002. R2=0.056; y= 98.6663+(−)0.3083x; p<0.001. (G) All samples: (Age: ≤12 months) N=1442; rho=−0.352, 95%CI=−0.396 to −0.306; p<0.001. R2=0.1032; y=42.6550+(−)2.0145x; p<0.001. (Age: >1 year to ≤18 years) N=2683; rho=0.348, 95%CI=0.315–0.381; p<0.001. R2=0.066; y=20.8087+1.4323x; p<0.001. (Age: >18 years) N=1071; rho=−0.052, 95%CI=−0.111 to 0.008; p=0.09. R2=0.003; y=52.1251+(−)0.07962x; p=0.090. α=0.05. Statistical analysis was made with linear regression test and Spearman's rank correlation test.
The sweat was collected following Gibson and Cooke's traditional method (1959) and the sweat chloride concentration was determined by chloridometry and the sweat sodium concentration by flame photometry. The sweat chloride levels were used to group the samples according to the CF diagnosis: (i) chloride<30mEq/L; (ii) chloride≥30mEq/L to <40mEq/L; (iii) chloride≥40mEq/L to <60mEq/L; (iv) chloride≥60mEq/L (group of patients with CF). The ST data was obtained from the medical records of the Pediatric Gastroenterology Laboratory and the Gastroenterology Center at the University Hospital.
The patients were divided into three age groups: (i) from birth to
Statistical analysis was carried out with linear regression test and Spearman's rank correlation test by the MedCalc software version 16.4.3. Significance level was set at 0.05 for all analyses. The power of the sample was greater than 80%.
The study was approved by the Ethics Committee of the University of Campinas (#474326).
As observed in Fig. 1G, the results from our total population agree with those reported by Traeguer and colleagues.2 The same correlation was observed for the groups of sweat chloride levels in mEq/L: (i) chloride<30mEq/L; (ii) chloride≥30mEq/L to <40mEq/L; (iii) chloride≥40mEq/L to <60mEq/L; (iv) chloride≥30mEq/L to <60mEq/L; (v) chloride<60mEq/L (Fig. 1A–E).
In contrast, as shown in Fig. 1F, CF patients show increased sweat chloride levels in the first year of life. These levels gradually reduced after the second year or life. This was not evidenced by Traeguer and colleagues,2 possibly due to the effect of sample dispersion of CF patients within the total sample.
It is important to note that sweat chloride levels tend to be lower among adults compared to children. Another important factor is the evidence that sweat chloride levels have intra- and inter-individual variability, even in patients with the same genotype in the Cystic Fibrosis Transmembrane Regulator (CFTR) gene.5
Such alterations should be further studied by measuring amounts of sweat chloride in CF patients and healthy individuals on a long-term basis.
One hypothesis that may explain decreased amounts of sweat chloride in sweat with increasing age is related to changes in the stability of the CFTR protein in healthy individuals and CF patients with borderline sweat chloride levels. On the other hand, for all subjects, reduced sweat chloride levels after 18 years of age may be a consequence of the aging process. Aging is a natural response, which causes physiological changes, including alterations in other chloride regulating channels and the action of modifier genes.
Therefore, studies should also be made on the factors that interfere with sweat chloride levels in different ages among healthy individuals and CF patients.
Ethics approval and consent to participateThe study was approved by the Ethics Committee of University of Campinas (#474326).
Authors’ contributionsAGF, FALM, JDR made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; were involved in drafting the manuscript and revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
AFR made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data.
Conflicts of interestAll the authors declare that they have no conflicts of interest.
Financial support from the following institutions: FALM: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), for sponsoring the researches #2011/12939-4, #2011/18845-1, #2015/12183-8 and #2015/12858-5; Fundo de Apoio à Pesquisa ao Ensino e à Extensão da Universidade Estadual de Campinas, for sponsoring the research #0648/2015. JDR: FAPESP, for sponsoring the research #2011/18845-1, #2015/12183-8 and Fundo de Apoio à Pesquisa ao Ensino e à Extensão da Universidade Estadual de Campinas, for sponsoring the research #17616/2015.
The group of cystic fibrosis: Elizete Aparecida Lomazi, Adyléia Dalbo Contrera Toro, Andressa Oliveira Peixoto, Carla Cristina de Souza Gomez, Carlos Emilio Levy, Carmen Sílvia Bertuzzo, Eulália Sakano, Gabriel Hessel, Ilma Aparecida Paschoal, Lucas Brioschi Morais, Maria Ângela Gonçalves de Oliveira Ribeiro, Maria de Fátima Servidoni, Mônica Corso Pereira; Roberto José Negrão Nogueira.




 1 year to ≤18 years) N=1827; rho=0.192, 95%CI=0.148–0.236; p<0.001. R2=0.039; y=17.1432+0.2749x; p<0.001. (Age: >18 years) N=230; rho=−0.048, 95%CI=−0.176 to 0.082; p=0.469. R2=0.002; y=23.7087+(−)0.01622x; p=0.538. (B) Chloride≥30mEq/L to <40mEq/L: (age: ≤12 months) N=52; rho=0.097, 95%CI=−0.181 to 0.360; p=0.495. R2=0.013; y=32.8243+0.07205x; p=0.427. (Age: >1 year to ≤18 years) N=340; rho=0.151, 95%CI=0.045–0.253; p=0.005. R2=0.021; y=33.4949+0.09905x; p=0.007. (Age: >18 years) N=244; rho=0.026, 95%CI=−0.100 to 0.151; p=0.692. R2<0.001; y=34.6330+0.003532x; p=0.758. (C) Chloride≥40mEq/L to <60mEq/L: (Age: ≤12 months) N=33; rho=0.025, 95%CI=−0.321 to 0.365; p=0.892. R2<0.001; y=48.4653+(−)0.09778x; p=0.748. (Age: >1 year to ≤18 years) N=278; rho=0.134, 95%CI=0.016–0.248; p=0.026. R2=0.018; y=46.4328+0.1699x; p=0.026. (Age: >18 years) N=343; rho=0.117, 95%CI=0.011–0.220; p=0.031. R2=0.014; y=46.9588+0.03910x; p=0.031. (D) Chloride≥30mEq/L to <60mEq/L: (Age: ≤12 months) N=85; rho=−0.049, 95%CI=−0.259 to 0.166; p=0.656. R2=0.005; y=39.4291+(−)0.1577x; p=0.5224. (Age: >1 year to ≤18 years) N=618; rho=0.210, 95%CI=0.134–0.285; p<0.001. R2=0.042; y=37.2303+0.3772x; p<0.001. (Age: >18 years) N=587; rho=0.017, 95%CI=−0.064 to 0.098; p=0.678. R2<0.001; y=42.3785+0.01182x; p=0.565. (E) Chloride<60mEq/L: (Age: ≤12 months) N=1263; rho=−0.246, 95%CI=−0.297 to −0.193; p<0.001. R2=0.078; y=21.5579+(−)0.4503x; p<0.001. (Age: >1 year to ≤18 years) N=2445; rho=0.336, 95%CI=0.300–0.370; p<0.001. R2=0.127; y=17.9823+0.968.9x; p<0.001. (Age: >18 years) N=817; rho=−0.01, 95%CI=−0.078 to 0.0586; p=0.775. R2<0.001; y=37.6153+(−)0.007263x; p=0.776. (F) Chloride≥60mEq/L: (Age: ≤12 months) N=179; rho=0.134, 95%CI=−0.013 to 0.275; p=0.073. R2=0.021; y=99.9960+0.8686x; p=0.058. (Age: >1 year to ≤18 years) N=238; rho=−0.287, 95%CI=−0.400 to −0.166; p<0.001. R2=0.085; y=107.6871+(−)1.6094x; p<0.001. (Age: >18 years) N=254; rho=−0.190, 95%CI=−0.306 to −0.068; p=0.002. R2=0.056; y= 98.6663+(−)0.3083x; p<0.001. (G) All samples: (Age: ≤12 months) N=1442; rho=−0.352, 95%CI=−0.396 to −0.306; p<0.001. R2=0.1032; y=42.6550+(−)2.0145x; p<0.001. (Age: >1 year to ≤18 years) N=2683; rho=0.348, 95%CI=0.315–0.381; p<0.001. R2=0.066; y=20.8087+1.4323x; p<0.001. (Age: >18 years) N=1071; rho=−0.052, 95%CI=−0.111 to 0.008; p=0.09. R2=0.003; y=52.1251+(−)0.07962x; p=0.090. α=0.05. Statistical analysis was made with linear regression test and Spearman's rank correlation test.' title='Correlation between levels of sweat chloride and age of subjects undertaking sweat tests, considering possibilities of groups by chloride concentrations. (A) Chloride<30mEq/L: (age: ≤12 months) N=1178; Spearman's coefficient rank correlation (rho)=−0.159, 95%CI=−0.214 to −0.103; p<0.001. Coefficient of determination R2=0.025; y=17.9905+(−)0.1643x; p<0.001. (Age: >1 year to ≤18 years) N=1827; rho=0.192, 95%CI=0.148–0.236; p<0.001. R2=0.039; y=17.1432+0.2749x; p<0.001. (Age: >18 years) N=230; rho=−0.048, 95%CI=−0.176 to 0.082; p=0.469. R2=0.002; y=23.7087+(−)0.01622x; p=0.538. (B) Chloride≥30mEq/L to <40mEq/L: (age: ≤12 months) N=52; rho=0.097, 95%CI=−0.181 to 0.360; p=0.495. R2=0.013; y=32.8243+0.07205x; p=0.427. (Age: >1 year to ≤18 years) N=340; rho=0.151, 95%CI=0.045–0.253; p=0.005. R2=0.021; y=33.4949+0.09905x; p=0.007. (Age: >18 years) N=244; rho=0.026, 95%CI=−0.100 to 0.151; p=0.692. R2<0.001; y=34.6330+0.003532x; p=0.758. (C) Chloride≥40mEq/L to <60mEq/L: (Age: ≤12 months) N=33; rho=0.025, 95%CI=−0.321 to 0.365; p=0.892. R2<0.001; y=48.4653+(−)0.09778x; p=0.748. (Age: >1 year to ≤18 years) N=278; rho=0.134, 95%CI=0.016–0.248; p=0.026. R2=0.018; y=46.4328+0.1699x; p=0.026. (Age: >18 years) N=343; rho=0.117, 95%CI=0.011–0.220; p=0.031. R2=0.014; y=46.9588+0.03910x; p=0.031. (D) Chloride≥30mEq/L to <60mEq/L: (Age: ≤12 months) N=85; rho=−0.049, 95%CI=−0.259 to 0.166; p=0.656. R2=0.005; y=39.4291+(−)0.1577x; p=0.5224. (Age: >1 year to ≤18 years) N=618; rho=0.210, 95%CI=0.134–0.285; p<0.001. R2=0.042; y=37.2303+0.3772x; p<0.001. (Age: >18 years) N=587; rho=0.017, 95%CI=−0.064 to 0.098; p=0.678. R2<0.001; y=42.3785+0.01182x; p=0.565. (E) Chloride<60mEq/L: (Age: ≤12 months) N=1263; rho=−0.246, 95%CI=−0.297 to −0.193; p<0.001. R2=0.078; y=21.5579+(−)0.4503x; p<0.001. (Age: >1 year to ≤18 years) N=2445; rho=0.336, 95%CI=0.300–0.370; p<0.001. R2=0.127; y=17.9823+0.968.9x; p<0.001. (Age: >18 years) N=817; rho=−0.01, 95%CI=−0.078 to 0.0586; p=0.775. R2<0.001; y=37.6153+(−)0.007263x; p=0.776. (F) Chloride≥60mEq/L: (Age: ≤12 months) N=179; rho=0.134, 95%CI=−0.013 to 0.275; p=0.073. R2=0.021; y=99.9960+0.8686x; p=0.058. (Age: >1 year to ≤18 years) N=238; rho=−0.287, 95%CI=−0.400 to −0.166; p<0.001. R2=0.085; y=107.6871+(−)1.6094x; p<0.001. (Age: >18 years) N=254; rho=−0.190, 95%CI=−0.306 to −0.068; p=0.002. R2=0.056; y= 98.6663+(−)0.3083x; p<0.001. (G) All samples: (Age: ≤12 months) N=1442; rho=−0.352, 95%CI=−0.396 to −0.306; p<0.001. R2=0.1032; y=42.6550+(−)2.0145x; p<0.001. (Age: >1 year to ≤18 years) N=2683; rho=0.348, 95%CI=0.315–0.381; p<0.001. R2=0.066; y=20.8087+1.4323x; p<0.001. (Age: >18 years) N=1071; rho=−0.052, 95%CI=−0.111 to 0.008; p=0.09. R2=0.003; y=52.1251+(−)0.07962x; p=0.090. α=0.05. Statistical analysis was made with linear regression test and Spearman's rank correlation test.'/>
1 year to ≤18 years) N=1827; rho=0.192, 95%CI=0.148–0.236; p<0.001. R2=0.039; y=17.1432+0.2749x; p<0.001. (Age: >18 years) N=230; rho=−0.048, 95%CI=−0.176 to 0.082; p=0.469. R2=0.002; y=23.7087+(−)0.01622x; p=0.538. (B) Chloride≥30mEq/L to <40mEq/L: (age: ≤12 months) N=52; rho=0.097, 95%CI=−0.181 to 0.360; p=0.495. R2=0.013; y=32.8243+0.07205x; p=0.427. (Age: >1 year to ≤18 years) N=340; rho=0.151, 95%CI=0.045–0.253; p=0.005. R2=0.021; y=33.4949+0.09905x; p=0.007. (Age: >18 years) N=244; rho=0.026, 95%CI=−0.100 to 0.151; p=0.692. R2<0.001; y=34.6330+0.003532x; p=0.758. (C) Chloride≥40mEq/L to <60mEq/L: (Age: ≤12 months) N=33; rho=0.025, 95%CI=−0.321 to 0.365; p=0.892. R2<0.001; y=48.4653+(−)0.09778x; p=0.748. (Age: >1 year to ≤18 years) N=278; rho=0.134, 95%CI=0.016–0.248; p=0.026. R2=0.018; y=46.4328+0.1699x; p=0.026. (Age: >18 years) N=343; rho=0.117, 95%CI=0.011–0.220; p=0.031. R2=0.014; y=46.9588+0.03910x; p=0.031. (D) Chloride≥30mEq/L to <60mEq/L: (Age: ≤12 months) N=85; rho=−0.049, 95%CI=−0.259 to 0.166; p=0.656. R2=0.005; y=39.4291+(−)0.1577x; p=0.5224. (Age: >1 year to ≤18 years) N=618; rho=0.210, 95%CI=0.134–0.285; p<0.001. R2=0.042; y=37.2303+0.3772x; p<0.001. (Age: >18 years) N=587; rho=0.017, 95%CI=−0.064 to 0.098; p=0.678. R2<0.001; y=42.3785+0.01182x; p=0.565. (E) Chloride<60mEq/L: (Age: ≤12 months) N=1263; rho=−0.246, 95%CI=−0.297 to −0.193; p<0.001. R2=0.078; y=21.5579+(−)0.4503x; p<0.001. (Age: >1 year to ≤18 years) N=2445; rho=0.336, 95%CI=0.300–0.370; p<0.001. R2=0.127; y=17.9823+0.968.9x; p<0.001. (Age: >18 years) N=817; rho=−0.01, 95%CI=−0.078 to 0.0586; p=0.775. R2<0.001; y=37.6153+(−)0.007263x; p=0.776. (F) Chloride≥60mEq/L: (Age: ≤12 months) N=179; rho=0.134, 95%CI=−0.013 to 0.275; p=0.073. R2=0.021; y=99.9960+0.8686x; p=0.058. (Age: >1 year to ≤18 years) N=238; rho=−0.287, 95%CI=−0.400 to −0.166; p<0.001. R2=0.085; y=107.6871+(−)1.6094x; p<0.001. (Age: >18 years) N=254; rho=−0.190, 95%CI=−0.306 to −0.068; p=0.002. R2=0.056; y= 98.6663+(−)0.3083x; p<0.001. (G) All samples: (Age: ≤12 months) N=1442; rho=−0.352, 95%CI=−0.396 to −0.306; p<0.001. R2=0.1032; y=42.6550+(−)2.0145x; p<0.001. (Age: >1 year to ≤18 years) N=2683; rho=0.348, 95%CI=0.315–0.381; p<0.001. R2=0.066; y=20.8087+1.4323x; p<0.001. (Age: >18 years) N=1071; rho=−0.052, 95%CI=−0.111 to 0.008; p=0.09. R2=0.003; y=52.1251+(−)0.07962x; p=0.090. α=0.05. Statistical analysis was made with linear regression test and Spearman's rank correlation test.' title='Correlation between levels of sweat chloride and age of subjects undertaking sweat tests, considering possibilities of groups by chloride concentrations. (A) Chloride<30mEq/L: (age: ≤12 months) N=1178; Spearman's coefficient rank correlation (rho)=−0.159, 95%CI=−0.214 to −0.103; p<0.001. Coefficient of determination R2=0.025; y=17.9905+(−)0.1643x; p<0.001. (Age: >1 year to ≤18 years) N=1827; rho=0.192, 95%CI=0.148–0.236; p<0.001. R2=0.039; y=17.1432+0.2749x; p<0.001. (Age: >18 years) N=230; rho=−0.048, 95%CI=−0.176 to 0.082; p=0.469. R2=0.002; y=23.7087+(−)0.01622x; p=0.538. (B) Chloride≥30mEq/L to <40mEq/L: (age: ≤12 months) N=52; rho=0.097, 95%CI=−0.181 to 0.360; p=0.495. R2=0.013; y=32.8243+0.07205x; p=0.427. (Age: >1 year to ≤18 years) N=340; rho=0.151, 95%CI=0.045–0.253; p=0.005. R2=0.021; y=33.4949+0.09905x; p=0.007. (Age: >18 years) N=244; rho=0.026, 95%CI=−0.100 to 0.151; p=0.692. R2<0.001; y=34.6330+0.003532x; p=0.758. (C) Chloride≥40mEq/L to <60mEq/L: (Age: ≤12 months) N=33; rho=0.025, 95%CI=−0.321 to 0.365; p=0.892. R2<0.001; y=48.4653+(−)0.09778x; p=0.748. (Age: >1 year to ≤18 years) N=278; rho=0.134, 95%CI=0.016–0.248; p=0.026. R2=0.018; y=46.4328+0.1699x; p=0.026. (Age: >18 years) N=343; rho=0.117, 95%CI=0.011–0.220; p=0.031. R2=0.014; y=46.9588+0.03910x; p=0.031. (D) Chloride≥30mEq/L to <60mEq/L: (Age: ≤12 months) N=85; rho=−0.049, 95%CI=−0.259 to 0.166; p=0.656. R2=0.005; y=39.4291+(−)0.1577x; p=0.5224. (Age: >1 year to ≤18 years) N=618; rho=0.210, 95%CI=0.134–0.285; p<0.001. R2=0.042; y=37.2303+0.3772x; p<0.001. (Age: >18 years) N=587; rho=0.017, 95%CI=−0.064 to 0.098; p=0.678. R2<0.001; y=42.3785+0.01182x; p=0.565. (E) Chloride<60mEq/L: (Age: ≤12 months) N=1263; rho=−0.246, 95%CI=−0.297 to −0.193; p<0.001. R2=0.078; y=21.5579+(−)0.4503x; p<0.001. (Age: >1 year to ≤18 years) N=2445; rho=0.336, 95%CI=0.300–0.370; p<0.001. R2=0.127; y=17.9823+0.968.9x; p<0.001. (Age: >18 years) N=817; rho=−0.01, 95%CI=−0.078 to 0.0586; p=0.775. R2<0.001; y=37.6153+(−)0.007263x; p=0.776. (F) Chloride≥60mEq/L: (Age: ≤12 months) N=179; rho=0.134, 95%CI=−0.013 to 0.275; p=0.073. R2=0.021; y=99.9960+0.8686x; p=0.058. (Age: >1 year to ≤18 years) N=238; rho=−0.287, 95%CI=−0.400 to −0.166; p<0.001. R2=0.085; y=107.6871+(−)1.6094x; p<0.001. (Age: >18 years) N=254; rho=−0.190, 95%CI=−0.306 to −0.068; p=0.002. R2=0.056; y= 98.6663+(−)0.3083x; p<0.001. (G) All samples: (Age: ≤12 months) N=1442; rho=−0.352, 95%CI=−0.396 to −0.306; p<0.001. R2=0.1032; y=42.6550+(−)2.0145x; p<0.001. (Age: >1 year to ≤18 years) N=2683; rho=0.348, 95%CI=0.315–0.381; p<0.001. R2=0.066; y=20.8087+1.4323x; p<0.001. (Age: >18 years) N=1071; rho=−0.052, 95%CI=−0.111 to 0.008; p=0.09. R2=0.003; y=52.1251+(−)0.07962x; p=0.090. α=0.05. Statistical analysis was made with linear regression test and Spearman's rank correlation test.'/>



