We evaluated the effect of weight loss (WL) on lung function (LF) in obese individuals who underwent bariatric surgery, and on asthma control, quality of life, LF, and controller medication in a sub-group of obese asthma (OA) patients.
Materials and methodsObese individuals who underwent bariatric surgery between July 2015 and July 2017 were included in this prospective longitudinal study. They were classified as OA or obese non-asthmatics (O-NA). LF was assessed preoperatively and 6–9 months postoperatively. In OA patients, asthma control, quality of life, and treatment step were evaluated. P < 0.05 was considered significant.
ResultsTwenty-six patients (OA: n = 8; O-NA: n = 18), 84.6% with class III obesity were enrolled. Preoperatively, OA patients showed worse values of LF parameters, with upper and lower airway CARAT scores of 6.1 ± 3.1 and 13.4 ± 4.1, respectively, and 75% were in step 4 of treatment. After WL, improvements in dynamic volumes, lung capacities, and total resistance were observed in both groups. Despite greater increases in OA patients, no significant differences were observed between groups. In OA patients, improvements in CARAT score of upper (3.9 ± 1.9, p = 0.017) and lower (4.2 ± 4.4, p = 0.027) airways, and in Asthma Life Quality scores (8.1 ± 5.6, p = 0.017) were observed along with a decrease (−1.8 ± 1.0, p = 0.017) in treatment step.
ConclusionsAll LF parameters improved after WL. Although the improvement was greater in OA patients, the difference between groups was not significant. Significant improvement from baseline in uncontrolled symptoms of OA patients and quality of life was observed after WL, along with a significant decrease in treatment step.
Approximately 30% of the population worldwide is overweight or obese, due to physical inactivity and increased intake of energy-dense foods that are high in fat.1 The percentage of overweight and obese individuals is estimated to double by the year 2020.1 The prevalence of morbid obesity is also estimated to increase to 11% by the year 2030.1 These factors make obesity, defined by the WHO as a body mass index (BMI) ≥30 kg/m2, the third largest social burden created by human beings, only surpassed by smoking and armed violence.1,2 According to the WHO, obesity is subdivided into three classes: class I (BMI = 30–34.9 kg/m2), class II (BMI = 35–39.9 kg/m2) and class III (BMI ≥ 40 kg/m2).2,3
Being overweight promotes metabolic and structural changes in the body; therefore, obese individuals present with several co-morbidities, such as cardiovascular disease, hypertension, type 2 diabetes mellitus, musculoskeletal disorders, and some forms of cancer.1,2,4,5 A higher BMI is also associated with a higher risk of developing obesity-related diseases.1,2
Respiratory disorders are one of the co-morbidities associated with obesity.4,6 The mechanism of such diseases is primarily mechanical, pertaining to the excess adipose tissue that covers the thorax and abdomen. The adipose tissue encumbers normal ventilation through limitation of two primary inspiratory movements: contraction of the diaphragm, which results in projection of the abdominal content in a downward and forward direction, and an increase in chest diameter due to the movement of the ribs.1,4,6–8 This results in stiffening of the total respiratory system and an increase in the mechanical work required to breathe.6,9
Additionally, adipose tissue has endocrine and paracrine functions, producing a large number of cytokines and bioactive mediators that generate a pro-inflammatory state in obese individuals associated with atopy, bronchial hyperactivity, and increased risk and severity of asthma, as well as endotypic modifications of asthma.1,4–8,10,11
Asthma is a common heterogeneous respiratory disease, characterized by chronic airway inflammation, with a substantially increasing prevalence in the later part of the 20th century.12,13 Recent data suggest that ≥15% of the general population in multiple countries suffer from asthma.12,13
The association between obesity and asthma is not well understood.1,2,5,7,11,14 Obese asthmatic (OA) patients have more symptoms, difficulty in controlling disease, more frequent and severe exacerbations, decreased response to both reliever and control medications, and worse quality of life.5,7,10–12,14
The obesity-related phenotype of asthma shows distinct characteristics and there is growing evidence that weight loss (WL) leads to an improvement in the control of asthma, quality of life, and lung function (LF), as well as a reduction in the use of medication.7,10,12,14 Asthma control is evaluated on the basis of the combination of the frequency and severity of day- and night-time symptoms, future risk of adverse outcomes such as exacerbations, use of rescue medication, and limitation of day-time activities.12 As the above-mentioned aspects constitute the aim of asthma management,12 a combination of both pharmacological and non-pharmacological therapies, such as WL, in the obesity-related phenotype, may be advantageous.
The treatment of obesity requires a multidisciplinary approach that includes behavioral modifications, a calorie-restricted diet, and physical exercise.10,14 However, bariatric surgery is the most effective intervention for producing sustained and significant WL.7,15 All studies describing the effect of bariatric surgery reported highly significant improvements in LF, control and exacerbations of asthma.7,14 Most of them include data from one year, but few demonstrate the effect of the significant improvement of all these outcomes associated with a significant reduction in controller medication.
Therefore, the aim of the present study was to evaluate the impact of WL on LF in obese individuals who had undergone bariatric surgery, and on control of asthma, quality of life, LF, and controller medication requirements in a sub-group of OA patients.
MethodsThe current work was a prospective, longitudinal study performed on obese patients undergoing bariatric surgery at the Surgical Obesity Treatment Unit of the General Surgery Department of Centro Hospitalar e Universitário de Coimbra (CHUC), Portugal, between July 2015 and July 2017. The CHUC Research Ethics Committee (CHUC-088-15) approved the study protocol.
Patients over 18 years of age who were scheduled to undergo bariatric surgery were invited to participate in the present study. Patients who consented to participate were questioned about their smoking habits, presence of respiratory disease, and medication use. This study included patients with no history of any respiratory disease rather than asthma or obstructive sleep apnea (OSA), who consented to participate.
All included patients signed an informed consent form. Classification of obesity was done according to the WHO criteria.3 Patients were divided into two groups based on the presence or absence of asthma: OA and obese non-asthmatics (O-NA). OA patients had a previous diagnosis of asthma according to Global Initiative of Asthma (GINA) diagnostic criteria in adults, confirmed retrospectively from their clinical charts, or showed a clinically significant response to bronchodilator medications, defined by an increase of >12% and >200 ml in forced expiratory volume in first second (FEV1) and/or forced vital capacity (FVC) from baseline, during assessment of LF before surgery.12 O-NA patients had no previous diagnosis of asthma by any physician and did not respond in a clinically significant manner to bronchodilator.
LF of all included patients was assessed the day before bariatric surgery (first day inward). In OA patients, control of asthma, quality of life and level of treatment were also evaluated on the same day.
LF (plethysmography and bronchodilation test) was evaluated in the Pulmonology Department of CHUC using plethysmograph MasterScreen™ Body, Vyaire, Germany, and the follow parameters were taken into account: FVC, FEV1, vital capacity (VC), total lung capacity (TLC), total lung resistance (Rtot), forced expiratory flow at 25–75% of FVC (FEF25–75%), flow when 75% of FVC had been exhaled (FEF75%). Control of asthma was evaluated using the Control of Allergic Rhinitis and Asthma Test (CARAT), and quality of life using Asthma Life Quality (ALQ) questionnaire. Level of treatment was assessed according to GINA treatment steps.12,16,17
CARAT is a brief self-administered Portuguese questionnaire divided into two sections: the first evaluates the symptoms of allergic rhinitis through four questions, in which a total >8 means good control; and the second section evaluates the symptoms of asthma in six questions, with good control defined as values >16.16 ALQ is a questionnaire comprised of 20 items with a Yes/No response, wherein all questions are equally weighted and the total score is the sum of all positive answers, ranging from 0 to 20.17
Patients underwent gastric bypass or vertical gastrectomy by laparoscopy. Before discharge, a new LF assessment was scheduled for all patients included after six to nine months, when patients returned to the hospital for follow-up (Surgical Obesity Treatment Unit protocol follow-up schedule). Control of asthma, quality of life, and GINA treatment step were re-evaluated in OA patients at the same time as the second LF assessment.
Patients who did not consent to continue to participate, those who did not undergo bariatric surgery, those who did not undergo LF assessment in the second follow-up, or those who died were excluded from the study.
Clinical (gender, age, weight, BMI, smoking habits, asthma control, quality of life, asthma treatment step) and functional parameters obtained were registered on a database. Statistical analyses were carried out using SPSS®, version 24.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). The data of the patients are presented using descriptive statistics: categorical data as frequencies (percentages) and continuous variables as mean and standard deviation (SD). Comparisons between the two groups, OA and O-NA, were performed using the Mann–Whitney U test, while comparisons between BMI, functional parameters, asthma control, quality of life, and asthma treatment step before and after bariatric surgery were performed using the Wilcoxon test. The normality of the distribution of variables was analyzed using the Kolmogorov-Smirnov test. P < 0.05 was considered significant.
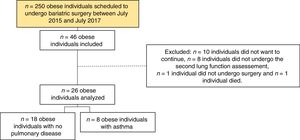
ResultsResults from 26 patients (Figure 1), with a median age of 44 years (SD = ±9.7), and who were predominantly female (n = 19, 73.1%) and non-smokers (n = 18, 69.2%) were analyzed. Before surgery, all patients were obese, with class III obesity (n = 22, 84.6%) in the majority of cases.
With respect to bariatric surgery, 65.4% of the patients underwent gastric bypass and 34.6% vertical gastrectomy surgeries. The initial mean value for BMI was 44.7 kg/m2 (SD = ±5.9), with a significant mean decrease of 11.3 kg/m2 (SD = ±3.8), p < 0.001, after 6–9 months of WL.
No significant differences were observed between genders (p = 0.935), age (p = 0.196), smoking habits (p = 0.849), or initial mean value (p = 0.531) and subsequent decrease (p = 0.892) of BMI between OA and O-NA patients (Table 1). Regarding the initial LF, only FEV1/FVC (p = 0.022), Rtot (p = 0.019), FEF25–75% (p = 0.030), and FEF75% (p = 0.047) were significantly worse in OA patients (Table 1).
General initial characteristics of the patients.
| Characteristic | Obese with no pulmonary disease n (%) or mean ± SD | Obese asthmatic n (%) or mean ± SD | P-value |
|---|---|---|---|
| Gender | |||
| Male/Female | 5 (27.8) / 13 (72.2) | 2 (25) / 6 (75) | 0.935 |
| Age (years) | 42 ± 8.3 | 48 ± 11.9 | 0.196 |
| Initial weight (kg) | 122.7 ± 23.8 | 112 ± 23.1 | 0.261 |
| Initial BMI (kg/m2) | 45.5 ± 6.1 | 43 ± 5.4 | 0.531 |
| BMI decrease (kg/m2) | 11.3 ± 3.5 | 11.3 ± 4.7 | 0.892 |
| Smoking habits | |||
| Non-smoker | 13 (72.2) | 5 (62.5) | |
| Current smoker | 4 (22.2) | 2 (25) | 0.849 |
| Former smoker | 1 (5.6) | 1 (12.5) | |
| Initial lung function | |||
| FEV1 /FVC | 82.7 ± 7.3 | 75.7 ± 7.0 | 0.022 |
| VC (%) | 103.6 ± 13.1 | 100.1 ± 12.7 | 0.807 |
| FVC (%) | 102.7 ± 13.4 | 99.7 ± 10.9 | 0.724 |
| Pre-bronchodilator FEV1 (%) | 100.5 ± 17.6 | 89.6 ± 12.5 | 0.102 |
| Post-bronchodilator FEV1 (%) | 104.7 ± 16.7 | 97.8 ± 16.6 | 0.311 |
| FEF75% (%) | 68.5 ± 36.2 | 38.5 ± 15.8 | 0.047 |
| FEF25-75% (%) | 83.6 ± 33.2 | 53.3 ± 20.4 | 0.030 |
| TLC (%) | 104.1 ± 10.3 | 102.5 ± 10.3 | 0.531 |
| RV (%) | 108.6 ± 18.2 | 110.2 ± 23.7 | 0.892 |
| Rtot (%) | 110 ± 35.2 | 162.2 ± 52.7 | 0.019 |
Legend: SD = standard deviation; BMI = body mass index; FEV1 = forced expiratory volume in first second; FVC = forced vital capacity; VC = vital capacity; FEF75% = forced expiratory flow when 75% of FVC has been exhaled; FEF25–75% = forced expiratory flow at 25–75% of FVC; TLC = total lung capacity; RV = residual volume; Rtot = total lung resistance.
O-NA patients showed an increase in TLC (137 mL, p = 0.199), as well as a significant increase in VC (199 mL, p = 0.005), FVC (249 mL, p = 0.011) and FEV1 (228 mL, p = 0.002) following WL. A significant improvement in Rtot (−0.090 kPa*s/L, p = 0.003) and increase of FEF75% and FEF25–75% of 316 mL (p = 0.002) and 358 mL (p = 0.014), respectively, were observed (Table 2).
Effect of weight loss on lung function after 6–9 months.
| Obese with no pulmonary disease | Baseline Mean ± SD | After 6 to 9 months Mean ± SD | P-value |
|---|---|---|---|
| VC (mL) | 3705 ± 821 | 3904 ± 853 | 0.005 |
| FEV1/FVC | 82.7 ± 7.3 | 84.1 ± 8.4 | 0.133 |
| FVC (mL) | 3577 ± 793 | 3826 ± 877 | 0.011 |
| FEV1 (mL) | 2963 ± 715 | 3191 ± 688 | 0.002 |
| FEF75% (mL/s) | 1299 ± 690 | 1615 ± 784 | 0.002 |
| FEF25–75% (mL/s) | 3123 ± 1381 | 3481 ± 1340 | 0.014 |
| TLC (mL) | 5522 ± 996 | 5659 ± 1069 | 0.199 |
| RV (mL) | 1826 ± 389 | 1776 ± 454 | 0.879 |
| Rtot (kPa*s/L) | 0.33 ± 0.11 | 0.24 ± 0.09 | 0.003 |
| Obese asthmatic | |||
| VC (mL) | 3223 ± 552 | 3491 ± 797 | 0.107 |
| FEV1/FVC | 75.7 ± 7.0 | 77.6 ± 8.0 | 0.208 |
| FVC (mL) | 3116 ± 558 | 3419 ± 817 | 0.040 |
| Pre-bronchodilator FEV1 (mL) | 2363 ± 490 | 2658 ± 643 | 0.017 |
| Bronchodilation in FEV1 (mL) | 186 ± 181 | 157 ± 130 | 0.483 |
| FEF75% (mL/s) | 654 ± 350 | 945 ± 466 | 0.018 |
| FEF25-75% (mL/s) | 1855 ± 806 | 2283 ± 835 | 0.012 |
| TLC (mL) | 5200 ± 1075 | 5859 ± 1162 | 0.036 |
| RV (mL) | 1938 ± 670 | 2354 ± 593 | 0.069 |
| Rtot (kPa*s/L) | 0.49 ± 0.16 | 0.32 ± 0.10 | 0.035 |
Legend: SD = standard deviation; VC = vital capacity; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; FEF75% = forced expiratory flow when 75% of FVC has been exhaled; FEF25–75% = forced expiratory flow at 25–75% of FVC; TLC = total lung capacity; RV = residual volume; Rtot = total lung resistance.
Before surgery, OA patients showed a mean CARAT score of 6.1 (SD = ±3.1) in the upper airways and 13.4 (SD = ±4.1) in the lower airways, with mean of total CARAT score of 19.6 ± 6.7. 25% of the patients were on Step 3 and 75% on Step 4 of the GINA treatment.
After WL, OA patients showed an increase in VC (268 mL, p = 0.107), as well as a significant increase FVC (303 mL, p = 0.040), FEV1 (295 mL, p = 0.017), and TLC (659 mL, p = 0.036). Significant improvement was also observed in FEF75% (291 mL, p = 0.018), FEF25–75% (428 mL, p = 0.012), and Rtot (−0.17 kPa*s/L, p = 0.035) (Table 2).
Improvement in LF was accompanied by a significant increase in CARAT score of the upper airways by 3.9 (SD = ±1.9, p = 0.017) and of the lower airways by 4.2 (SD = ±4.4, p = 0.027), as well as a significant mean decrease of 1.8 (SD = ±1.0, p = 0.017) in the GINA treatment step. After surgery, all OA decrease the step of treatment: 37.5% were on Step 3, 12.5% were on Step 2, and 50% were on Step 1 of the GINA treatment, with a mean of total CARAT score was 27.6 ± 1.5. The initial mean value for ALQ was 9.6 (SD = ±5.3), which improved significantly with WL to 4.1 (SD = ±2.6, p = 0.017).
The results showed no significant difference in improvement of LF between OA and O-NA patients with respect to VC (p = 1.000), FVC (p = 0.849), FEV1 (p = 0.495), TLC (p = 0.54), FEF75% (p = 0.849), FEF25–75% (p = 0.567), or Rtot (p = 0.397).
DiscussionThe present study demonstrates an early significant improvement in LF, control of asthma, and quality of life together with a significant reduction in controller medication, in OA after WL.
Before surgery, symptoms of allergic rhinitis and asthma were not controlled in OA patients despite step 4 or 5 of the GINA treatment. Data published by Esteban-Gorgojo et al. showed that obesity-related asthma is predominantly non-eosinophilic and, although obesity can be either a cause or consequence, it seems more plausible that it is caused by increased asthma severity.18 In addition to the mechanical effect of excessive fat accumulation in the thoracic and abdominal cavities, obesity induces a state of low-grade inflammation.6,7,11,15,19,20 In OA patients, overproduction of leptin in the adipose tissue stimulates the production of pro-inflammatory mediators, such as TNF-α, IL-6, and IFN-γ, which can all cause bronchial hyperactivity, pulmonary inflammation, and a poor response to inhaled corticosteroids.5–8,15,18,20,21 IL-6 has also been associated with smooth muscle proliferation and epithelial damage in the airways, leading to its remodeling.15 Abnormalities in surfactant function leading to increased alveolar instability and collapse, as well as increased oxidative stress with reduced bioavailability of the endogenous bronchodilator NO, also have a negative effect on asthma outcomes.7,21 In the obesity-related phenotype of asthma a worse response to standard controller and reliever medications may also be due to associated comorbidities, such as OSA and gastroesophageal reflux disease (GERD).5,8,12,14,22,23 Obesity is a well-established risk factor for the development of GERD that, in turn, hinders the control of asthma.1,5,8,12,23 Some studies have reported that the asthma medication required before bariatric surgery is strongly influenced by BMI and GERD.24
After surgery, a significant decrease was observed in the mean value of BMI along with a significant improvement in the control of asthma and quality of life, which was accompanied by a significant decrease in the step of GINA treatment. Previous studies have also reported an improvement in asthma outcomes and a decrease in the use of medications with WL.10,24 Guerron et al. reported that this reduction was observed as early as 30 days post-bariatric surgery and progressed over time, regardless of the type of procedure.24 However, as far as we know, very few studies assessed all these outcomes together with these significant results within a short period of time. It must be noted that after 6–9 months of follow-up, despite the fact that half of them were on Step 1 of GINA treatment and none needed controller medication on Step 4, they achieved a good control. The primary goal of asthma treatment is to control symptoms with minimal side effects.12 It must be understood that all pharmacological therapies may be associated with side effects, which in the case of asthma, are higher when a high dose of inhalational corticosteroids are used, as observed in more severe asthmatic individuals, such as OA.12 It may be speculated that WL could lead to a reduction in bronchial inflammation and consequently, better control of asthma and quality of life, even with a reduction in the step of GINA treatment. However, better control of comorbidities, such as GERD and OSA, may also play an important role. Regarding the quality of life, the worst values before surgery may also be related to the greater limitations in the activities of daily living with more symptoms following mild efforts, as a consequence of being overweight.
The mechanism by which obesity causes disorders of LF has not been completely established.4 A review conducted by Melo et al. demonstrated a reduction in FEV1, FVC, VC, and TLC in obese individuals and some studies reported such changes even in cases with a minimal degree of obesity.4,25 However, it must be noted that extremely obese individuals may have normal LF, as observed in O-NA patients prior to surgery in the present study.4,6 A review conducted by Cheryl et al. reported that despite many studies describing an association between an increase in BMI and decreased TLC, the effects of obesity on TLC are modest and this LF parameter is usually maintained within a normal range even in patients with severe obesity.6,9 Similarly, the authors have reported that although FEV1 and FVC tend to decrease with an increase in BMI, the effects of obesity are minimal and the LF parameters mentioned are usually within the normal range in healthy obese adults.6,9
Preliminary studies suggested a reduction in the diameter of small airways; however, the results are not consistent.6,9,25 In the present study, O-NA patients showed no impairment in the baseline dynamic volumes of small airways. Similarly, Cheryl et al. reported an increase in lung resistance in obese individuals, indicating that the caliber of the airway is reduced throughout the tidal breathing cycle.9 However, the authors also stated that specific airway resistance, adjusted for the lung volume at which the measurement was made, was within the normal range, which could explain the reason for the normal baseline Rtot value in O-NA patients in the present study.9
Although obesity has a tendency to induce restrictive disorders, obese patients breathe at low lung volumes and the tidal volume is typically approximately the same as the closing volume.23 The consequent repeated opening and closing of peripheral airways may lead to rupture of alveolar attachments to bronchioles, uncoupling the airways from the retractile forces of the parenchyma and worsening the limitation of airflow.23 FEF75% and FEF25–75% were described as being more reproducible and sensitive to detecting limitations in the small airways than FEV1.26 However, when FVC and TLC are affected by disease, forced expiratory flows are measured in different lung volumes than in healthy subjects, making the use of predicted values calculated from healthy subjects problematic.26 Thus, the reduction, in the absence of airway obstruction, may result from reduced lung volume rather than from airway disease.26 This may explain the reduced FEF75% and FEF25–75% in OA patients in the present study and the significant improvement with WL.
Despite the fact that obesity chiefly affects lung volume, with the preservation of dynamic volumes, improvement was observed in all capacities and dynamic volumes in the population included in the present study, with significant changes in FEV1, FVC, FEF75%, and FEF25–75%, as well as Rtot, in both groups after WL.1 The improvement was higher in OA patients; however, the difference between the groups was not significant. The improvement in TLC with WL supports the hypothesis concerning the mechanical effect of adipose tissue on LF in obese individuals.9 Additionally, a significant increase in TLC was observed only in OA patients, refuting the hypothesis that the parenchyma of these patients were more compliant than in O-NA—, causing them to collapse more easily due to lung compression.21 Improvement in FEV1, FVC, FEF75%, and FEF25–75%, even in O-NA patients, suggested the relative effect of WL on the diameter of airways. Similarly, a significant decrease in Rtot was probably due to a reduction in the stiffness of the chest wall with WL and recruitment of airways that were previously closed due to compression by excess adipose tissue. In O-NA patients, the decrease in Rtot supports the hypothesis that the increase in the value with obesity could be attributed to a reduction in lung volume rather than airway obstruction.9
The sample in the present study is comprised of patients who underwent bariatric surgery; however, in general, not all OA patients meet the criteria for surgical intervention. Previous studies have evaluated the effect of nonsurgical WL in patients with asthma and reported a significant improvement in LF, use of rescue medications, and frequency and severity of symptoms, as well as the number of exacerbations.10,15 A study by Scott et al. suggested that WL of 5–10% may be associated with significant improvements in asthma outcomes.27 The superiority of surgical WL lies in the higher rate of success, larger reduction in BMI (±15 kg/m2), and lasting results.15
The present study has some limitations. First, the sample size was small reducing the ability to analyze certain effects, such as the differences in the effect of WL on pre-existing asthma complicated by the subsequent development of obesity and symptoms of asthma that developed as a consequence of obesity. On the other hand, BMI was considered a measure of obesity, which has some limitations, such as the inability to describe the distribution of fat. Fat distribution has distinct implications concerning normal physiological changes in the respiratory system, as well as on metabolic inflammation.4,6,25 Control of comorbidities such as OSA and GERD were not assessed at baseline and after WL despite the fact that control of these factors may contribute to better control of asthma and improved quality of life.8,22,28 Additionally, levels of serum eosinophils, exhaled NO, or airway responsiveness to methacholine were not assessed prior to and 6–9 months after surgery. Therefore, conclusive information cannot be provided pertaining to changes in inflammatory status that may contribute to symptoms of asthma.
ConclusionsWeight loss proved to be effective in the improvement of LF in both OA and O-NA patients. Additionally, significant improvement in the control of asthma and quality of life was recorded in OA patients, which was accompanied by a significant decrease in the GINA treatment step. Thus, it is important to implement healthcare programs for the obese population, in order to improve LF and, consequently, quality of life. Regarding OA patients, the therapeutic approach should combine pharmacological therapies with WL, rather than primarily focusing on disease control by stepping up asthma therapy.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interestNone.