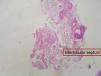
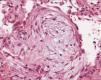
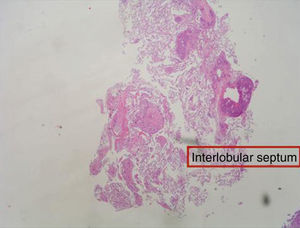
The diagnosis of diffuse parenchymal lung disease (DPLD) may require invasive procedures after all noninvasive tools have failed. The clinical context in which these diseases develop and the radiological patterns are crucial for defining the timing and the methods to be used. After the introduction in clinical practice of High Resolution CT scan (HRCT), the evaluation of imaging patterns, along with the immunological status of the patient and the clinical course of the disease (acute vs. chronic) seem to be crucial in choosing the best diagnostic procedure.1,2 In the immunocompromised patients who develop DPLD, a prompt diagnosis is essential for a better survival. In this circumstance, bronchoalveolar lavage (BAL), especially when the bronchoscope is guided by HRCT scan findings performed a few hours before, is generally of value in diagnosing opportunistic infections, alveolar proteinosis, alveolar hemorrhage and capillaritis, lymphomatous or leukemic lung infiltration, carcinomatous lymphangitis, disseminated hematogenous lung metastases, hypersensitivity pneumonitis, eosinophilic pneumonitis or diffuse alveolar damage due to drugs or radiation. In these cases, when a conclusive diagnosis is not obtained by BAL fluid analysis or in most immunocompetent patients who develop DPLD, transbronchial lung biopsy (TBLB) or even open lung biopsy could be considered. The TBLB forceps makes it possible to obtain lung tissue through the bronchial routes and the specimens so obtained usually represent from the centrilobular regions.3,4 Therefore, the disorders that are centered around terminal and respiratory bronchioles [respiratory bronchiolitis (RB), tuberculosis, lobular infectious pneumonia, cellular bronchiolitis] or significantly involve these structures (organizing pneumonia) or are distributed along the lymphatic routes (sarcoidosis, carcinomatous lymphangitis) may be frequently sampled by the forceps.4,5 In 1965, Andersen and colleagues at the Mayo Clinic described for the first time the rigid bronchoscopic technique of TBLB6 and in the subsequent trials they reported that lung tissue was obtained in 84% of patients with DPLD by using the technique of TBLB.7 The introduction of the flexible bronchoscope in the late 1960s increased the popularity of the technique and demonstrated that TBLB with the flexible instrument may be obtained with minimal mortality and morbidity. Currently TBLB is a well-established diagnostic technique used by almost all bronchoscopists, and the flexible bronchoscope is used almost exclusively. The main complication of TBLB is bleeding; less frequent complications are pneumothorax, hypoxemia, or cardiac arrhythmias during the procedure. Bleeding occurs to some degree in virtually all TBLB procedures and it is the main limiting factor in obtaining more or larger biopsy samples. Bleeding is a major concern to the bronchoscopist because of the limited options available to manage excessive bleeding through the flexible bronchoscope. The suction channel of the typical bronchoscope is only 2mm in diameter, and the volume of blood that can be suctioned through the channel is therefore limited. However, to minimize the consequence of hemorrhage by TBLB, it is possible to perform the procedure using the rigid bronchoscopy and placing a non inflated Fogarty balloon and a rigid aspirator (4mm in diameter) in lobar bronchus near the biopsy segment. The Fogarty balloon can be promptly inflated in case of bleeding. In this way it is much easier to control the bleeding complications.8,3 The main utility of the TBLB rests in the possibility of making a specific diagnosis in a patient with DPLD and avoiding a surgical lung biopsy. Some studies have demonstrated that the morphologic findings of interstitial inflammation and fibrosis in TBLB (such as in the diagnosis of idiopathic interstitial pneumonia) appeared to have little relevance to subsequent clinical course.9 While the monomorphous histological patterns (organizing pneumonia, eosinophilic pneumonia, diffuse alveolar damage with or without eosinophils, alveolar hemorrhage with or without capillaritis, alveolar proteinosis) are easily identifiable in small lung fragments (but only in agreement with clinical, radiological and other laboratory data),4,10 the tiny specimens obtained by TBLB may not be sufficient to define the histological pattern (usual interstitial pneumonitis, desquamative interstitial pneumonitis, nonspecific interstitial pneumonitis, fibrosing type). However, from the morphological point of view, generous TBLB may also provide good specimens for a diagnosis of a pattern called nonspecific interstitial pneumonia (NSIP), cellular variant in specific clinical settings such as polymyositis-dermatomyositis-related interstitial lung disease, drug toxicity ore in cases of unknown etiology11 although surgical lung biopsy is considered the best way to obtain enough tissue to demonstrate this pattern. The question of which kind of biopsy should be performed to diagnose DPLD has a long and controversial history. Certainly, the surgical lung biopsy provides substantially more material for pathological study, but the procedure requires general anesthesia, and one or more days in the hospital with a chest tube in place. Moreover, some reports have shown that this surgical procedure may be associated with increased mortality in certain states of the disease. In the experience of many interstitial lung disease centers across the world the median mortality is 4-6% within 30 days following surgical lung biopsy for UIP12,13 but biopsy performed at the time of acute exacerbation (AE) resulted in higher 30-day mortality (28.6%) compared to non-AE.14 Therefore, in recent years some studies have sought to increase the diagnostic yield of transbronchial lung biopsy in diagnosis of DPLD to avoid the surgical lung biopsy. In this respect, Berbescu et al15 reviewed 22 patients with known UIP (21 cases diagnosed by surgical biopsy, 1 case diagnosed by clinical and radiologic features) and then retrospectively examined transbronchial biopsies (TBLB) from these patients. They concluded that a diagnosis of UIP was possible on the TBLB in seven subjects, and in two others that the findings were consistent with UIP. As a rationale for the use of TBLB, the authors reported the high mortality within 30 days following surgical lung biopsy for UIP. However in a critical editorial Churg and Schwarz dampened this enthusiasm and suggested that “the collective wisdom be followed and that TBLB should not be used to diagnose IPF/UIP” because the morphologic findings of interstitial inflammation and fibrosis in TBLB appeared to have little relevance to the subsequent clinical course.16 However that has not stopped the tenacity of other researchers in assessing the improved diagnostic yield using non-surgical methods for the histological diagnosis of fibrosing diffuse lung disease. In a study,8 we reported a significant diagnostic yield and/or better preserved specimens (Fig. 1, 2) when TBLB were performed under general anesthesia with rigid bronchoscope using larger forceps (called Jumbo forceps) (Fig. 3) and without pulling them through the biopsy channel. Then a new way of obtaining TBLB using flexible cryoprobes was recently proposed (Fig. 4a, b, c). The cryosurgical equipment operates by the Joule-Thompson effect, which dictates that a compressed gas released at high flow rapidly expands and creates a very low temperature. The advantage of the cryoprobe is that large pieces of tissue can be extracted with the freeze-thaw cycle. The first published data17,18 indicate that, especially in idiopathic interstitial pneumonia, transbronchial cryobiopsy could add important information. The size of the samples obtained seems to increase the diagnostic yield significantly compared to forceps biopsies. Transbronchial cryobiopsies are much larger than conventional forceps biopsies but smaller than surgical lung biopsies. Despite having a potentially large defect in the lung parenchyma, the occurrence of pneumothorax and bleeding was low compared to data from forceps biopsies. These data, therefore, indicate that the TBLB may have even greater potential role in the diagnostic algorithm of DLDL (even in its fibrosing variants).
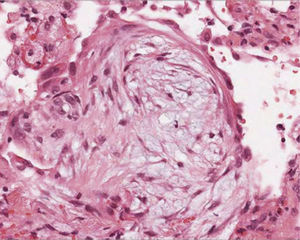
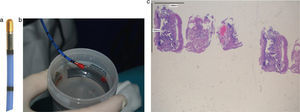
(a) A flexible cryoprobe is shown. The metal tip, can be visualized under fluoroscopic control. (b) Transbronchial lung biopsy obtained by flexible cryoprobes with lung sample attached to the metal tip. (c) Hematoxylin Eosin (low power). Large specimens showing a bronchiole and lung parenchyma (interstitial inflammation; preserved lung architecture).
Furthermore, when TBLB was introduced into clinical practice, a positive biopsy by the TBLB technique was defined as diagnostic histology, histology that supported a diagnosis, or histology consistent with the final diagnosis. Indeed, most of the published reports have focused on the diagnostic accuracy of histologic analysis of TBLB in patients with diffuse lung disorders. In contrast there were no data on how TBLB results are used by clinicians. In a very interesting study, Ensminger and Prakash19 evaluated if the results of the TBLB were clinically useful in the management of patients with diffuse pulmonary disorders. In the study TBLB was considered useful if: (a) it resulted in a specific clinical diagnosis, (b) a specific management decision was made based on the biopsy result, or (c) certain pathologic processes were excluded on the basis of the biopsy result and other clinical information. The results indicated that TBLB is a clinically useful test in approximately 75% of procedures, certainly not a negligible percentage.
In view of the above data, we believe that the use of TBLB in the diagnosis of DLDL is useful and safe, but certainly still too underestimated and undervalued by most clinicians. We believe that a future topic in the study of DPLD should be a greater implementation of the techniques of immunohistochemistry, molecular biology and microarray to be applied even on tiny samples obtained from the TBLB to increase the diagnostic yield and reduce the need to refer to surgical lung biopsy for diagnosis.
Conflict of interestsThe authors have no conflicts of interest to declare.