Endometriosis is characterized by the presence of endometrial-like glands and stroma outside the uterine cavity. It affects approximately 6–10% of reproductive-aged women. The most common site of endometriosis outside of the abdominopelvic cavity is the thorax. The main manifestations of endometriosis in the thorax include catamenial pneumothorax, catamenial hemothorax, catamenial hemoptysis with ground-glass opacities and pulmonary nodules, collectively known as thoracic endometriosis syndrome (TES).1 The pathologic identification of endometrial tissue is not always feasible, and diagnosis is usually based on the presence of clinical signs and symptoms with “catamenial” characteristics, combined with imaging.2,3 Several tests, such as chest CT and MRI, can be useful for the diagnosis of thoracic endometriosis. Moreover, thoracic imaging may additionally identify pneumomediastinum, pneumoperitoneum, ground-glass opacities, and thin-walled cavities within the lung parenchyma.1
Diffuse cystic lung disease (DCLD) is characterized by the presence of cysts in more than one lung lobe, usually bilaterally. Differential diagnosis of DCLD includes neoplastic, inflammatory, and infectious etiologies, such as lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, lymphocytic interstitial pneumonia, Birt–Hogg–Dubé disease and Ehlers–Danlos syndrome.4
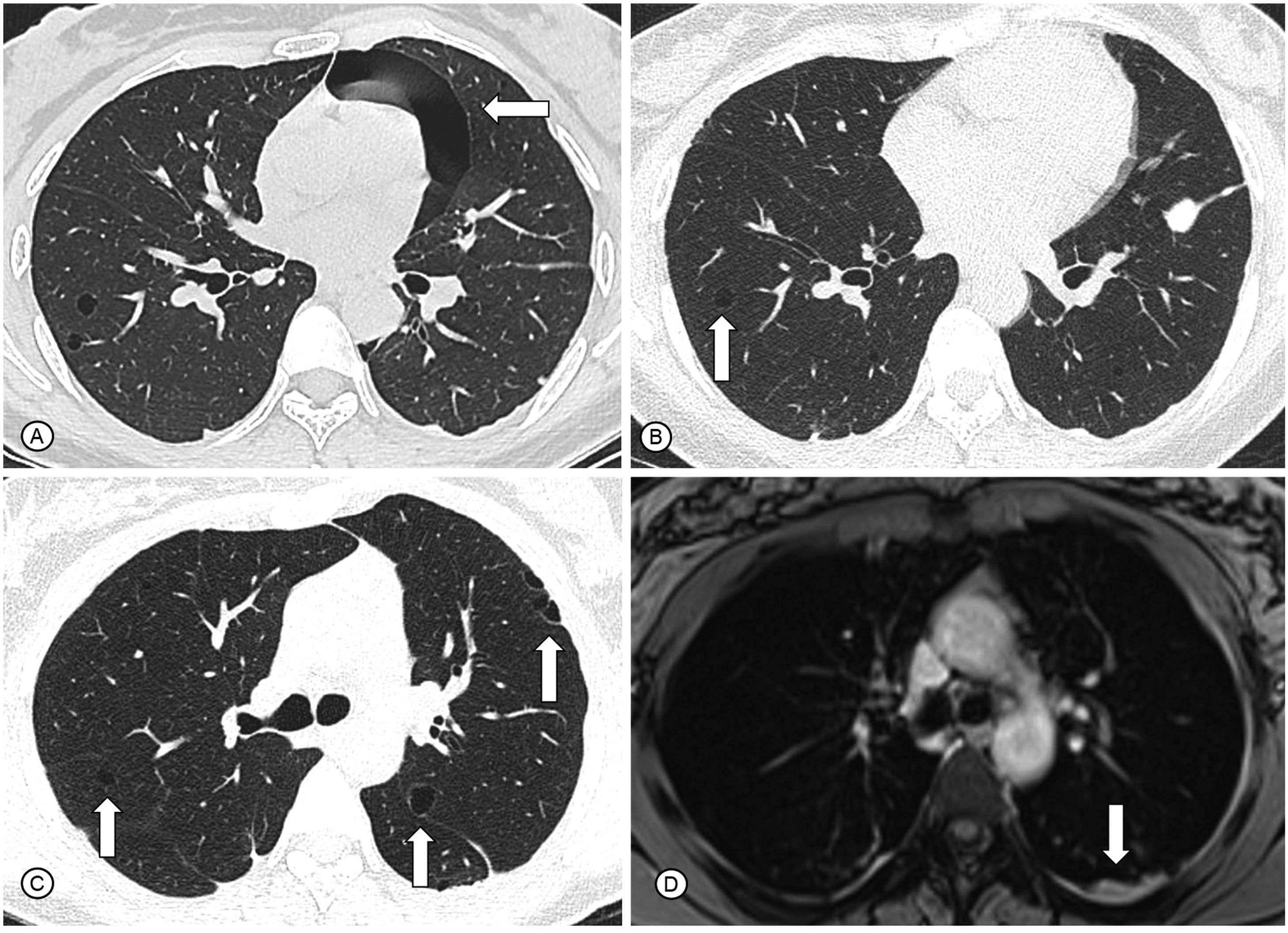
A 34-year-old woman was admitted to our clinic with complaints of exertional dyspnea for 4 years associated with chronic pelvic pain. Since the onset of symptoms, she had three spontaneous pneumothoraces, two in the left lung and one in the right lung that were drained and had complete resolution (Fig. 1A). Bilateral pleurodesis with talc was performed in both the hemithoraces. She had no comorbidities and no history of smoking. She had used chronic combined oral contraceptives. Respiratory examination was normal, and there was no hypoxemia at rest in room air. Chest CT performed one month after the last pneumothorax showed small, regular, and thin-walled cysts in both lungs, without other manifestations such as pleural effusion or pneumothorax (Fig. 1B and C). Pulmonary function tests demonstrated a proportional reduction in FEV1 and FVC (FVC = 44% of predicted), with a moderate reduction in the diffusion capacity of the lungs for carbon monoxide (61% of predicted). Laboratory tests were normal, including negative autoantibodies such as antinuclear antibody, rheumatoid factor, anti-Ro/SSA, and La/SSB. Chest MRI showed slight bilateral posterior pleural thickening with an intermediate signal on T2, that may correspond to the blood content (Fig. 1D). Abdominal and pelvic MRI showed thickening in the uterine retrocervical region with hypersignal on T1 and fat suppression, that may correspond to hemorrhagic areas suggestive of endometriosis. No evidence of renal angiomyolipoma or lymphangioleiomyoma was found. After multidisciplinary discussion, right lung segmentectomy was performed to establish the diagnosis. Histopathological analysis demonstrated endometrioid-type stromal cells around well-represented subpleural cyst that showed evident mural proliferation, with immunohistochemistry negative for melanoma marker antibody (HMB45) and beta-catenin. However, it was positive for Wilm's tumor 1 gene (WT1), CD10, and estrogen receptor, which confirmed the diagnosis of thoracic endometriosis (Fig. 2). CD10 is considered a sensitive immunohistochemical marker for (normal) endometrial stroma and endometrial stromal neoplasms. The WT1 gene has an essential role in the regulation of the development of the urogenital tract. It is expressed both in normal cells of the genital tract (granulosa, myometrium and endometrial stroma) and in tumors of adnexal origin and serous subtype. There was a relationship between spontaneous pneumothorax and the menstrual cycle, with most events occurring in the menstrual phase without any other catamenial manifestations. After a multidisciplinary approach involving a pulmonologist, gynecological surgeon, and thoracic surgeon, treatment with a GnRH analog (goserelin) was started. After treatment for 3 months, the patient was asymptomatic and the cysts remained stable on chest CT.
In (A), axial CT scan demonstrates pulmonary cysts and a pneumothorax (white arrow) during the follicular phase of the menstrual cycle. In (B) and (C), axial reconstructions of CT scans demonstrate small, regular and thin-walled cysts in both lungs (white arrows). In (D), chest MRI shows slight bilateral posterior pleural thickening, with an intermediate signal on T2, that may correspond to blood content (white arrow).
Legend: CT: computed tomography; MRI: magnetic resonance imaging.
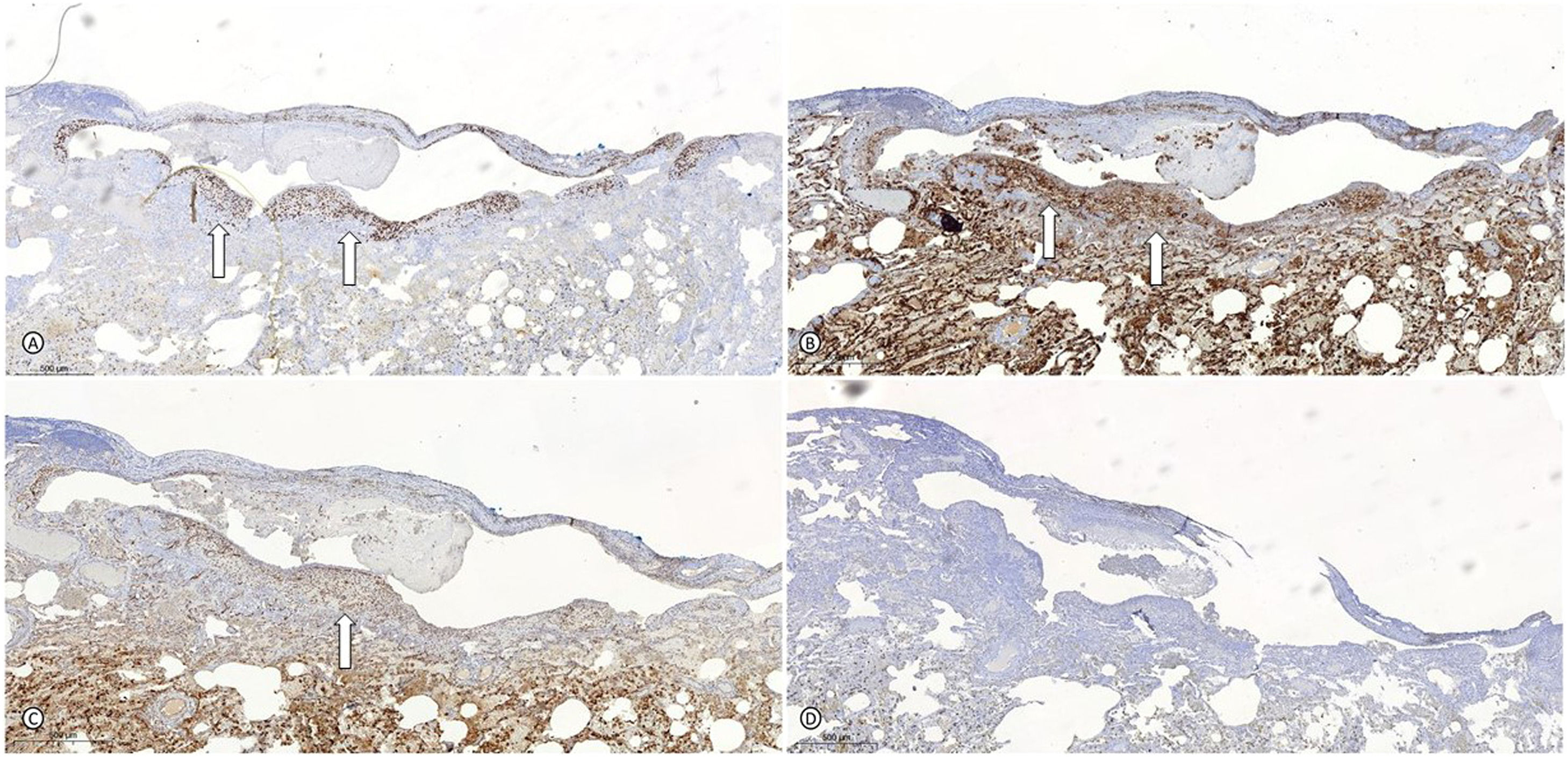
Right lung segmentectomy demonstrating endometrioid-type stroma cells around subpleural areas of cystic appearance stained positively for the estrogen receptors (A), CD10 (B), and WT1 (C) (white arrows) and negatively for HMB45 (D), that confirmed the hypothesis of thoracic endometriosis.
Legend: HMB45: melanoma marker antibody; WT1: Wilm's tumor 1 gene.
To the best of our knowledge, this is the first report of thoracic endometriosis presenting with DCLD. TES, although rare, may cause uncomfortable and even disabling manifestations, leading to a worsening of quality of life. The majority of TES cases involve solely the diaphragm, and laparoscopic excision is usually sufficient for complete disease control.5 The pathophysiology of the cysts in our case was not completely defined. However, some authors postulate that a local intrapulmonary endometrial implant swells during menstruation, enlarging in size, and constricting the bronchus. Thus, creating a check valve obstruction, leading to cyst formation.6 Additionally, cyclic endometrial shedding in the lungs causes destruction of the lining of the alveolar epithelial cells. This repeated destruction of alveolar architecture may lead to the formation of bullae. Although parenchymal cysts were not demonstrated in the histopathological analysis, we considered that it was probably associated with endometriosis, due to the fact that there was no other potential etiology for its occurrence and to the confirmation of such etiology in the subpleural cysts.
As with pelvic diseases, the first-line therapy for TES is drug treatment with the goal of suppressing ovarian steroid hormone production.1,2 Alternatives to GnRH analogs include oral contraceptives, progestins, danazol, and aromatase inhibitors, and most recently, GnRH antagonists. Minimally invasive surgical techniques are becoming increasingly used and may be considered for the evaluation and treatment of thoracic endometriosis, especially in cases of localized and focal diseases.1,2 However, systemic treatment was considered more appropriate for our patient.