The endobronchial ultrasound (EBUS) scope has been increasingly used in the gastrointestinal tract (EUS-B). Scientific data proves its efficacy and safety to provide a complete lung cancer staging, when combined with EBUS-TBNA, and in the diagnosis of para-esophageal lesions. There are multiple barriers to start performing EUS-B but probably the most important ones are related to knowledge and training, so new operators should follow a structured training curriculum. This review aims to reflect the best current knowledge regarding EUS-B and provide a road map to assist those who are incorporating the technique into their clinical practice.
The development of the endobronchial ultrasound (EBUS) scope and its incorporation into clinical practice was a breakthrough in XXI century respiratory medicine. A simple and elegant procedure, it has permitted safe diagnosis and/or staging of pulmonary and mediastinal disease, avoiding more invasive and costly exams.
As knowledge grew, new indications arose and experienced centers published data concerning the combined use of EBUS and EUS (endoscopic ultrasound through the esophagus) needle aspiration (NA) to correctly stage non-small cell lung cancer patients.1,2 Although successful, this approach had several limitations as it required a proficient endoscopist – initially only a small group of chest physicians worldwide had enough expertise to use the EUS scope – dedicated equipment, additional time and sometimes different settings, which taken all together contributed to increased costs and to delayed implementation.
Successful transesophageal and gastric use of the EBUS scope (EUS-B) was first reported in 2007.3 In 2010, two landmark papers proved that the dual use of EBUS, through the tracheal-bronchial tree and the esophagus was feasible and could be performed sequentially in the same setting, by one operator and with a high diagnostic yield for lung cancer staging.4,5 This strategy has shown that, potentially, most of the previous limitations can be overcome. These publications were soon followed by certification for EBUS scopes to be used in the upper gastrointestinal tract.
In the last few years, EBUS has disseminated from academic centers to community-based hospitals and is now an important equipment in most bronchoscopy units. Gradually, more chest physicians are also inserting the EBUS scope into the esophagus due to its advantages and encouraged by their familiarity with the equipment. EUS-B-NA makes a more complete assessment of the mediastinum possible when added to EBUS-TBNA. It provides nearly complete access to all relevant lymph nodes for staging lung cancer; permits the diagnosis of para-esophageal mediastinal and lung lesions which cannot be accessed through the tracheo-bronchial tree; permits access to the lower mediastinal lymph node stations (e.g. stations 8 and 9) and sub-diaphragmatic lymph nodes; and in comparison to EBUS it may offer an easier alternative for puncturing challenging lymph nodes in certain patients (e.g. stations 2L and 4L). Additionally, EUS-B-NA is usually a well-tolerated procedure under conscious sedation, achieved by lower doses of sedatives and anesthetics and is associated with less cough and oxygen desaturation when compared to EBUS, which is an important consideration in patients with compromised lung function.6
Nevertheless, there are multiple barriers to starting performing EUS-B-NA and the most important ones are related to knowledge and training. A few publications provide instructions on how to perform EUS but they are not dedicated to EUS-B.7–9 The present review aims to reflect the best current knowledge regarding this technique, focusing on recent guidelines, and to provide a road map to assist those who want to integrate EUS-B into their clinical practice.
When to do EUS-B?Malignant diseasesLung cancerRegarding the correct diagnosis and staging of lung cancer patients, tissue confirmation is still mandatory to select the best therapy and to enhance outcomes.10 In addition, enlarged lymph nodes in the mediastinum and/or the pulmonary hilum are always suspicious for malignancy and require further evaluation.
Histological biopsies obtained by mediastinoscopy have been the diagnostic gold standard for a long time but this surgical approach is invasive and associated with morbidity and additional costs.11,12 Therefore, minimally invasive techniques have been developed. EUS-NA, the endoscopic sampling of cytological specimens from the mediastinum through the esophagus, is the older technique, largely performed by gastroenterologists. With the EUS-scope the left mediastinum and the upper part of the abdomen can be assessed. In contrast, EBUS provides an endoscopic access to the upper mediastinal and the hilar lymph node stations, but distal paraesophageal lymph nodes or structures below the diaphragm cannot be reached via the endobronchial way. Both techniques represent a valuable alternative to surgical staging (Fig. 1).
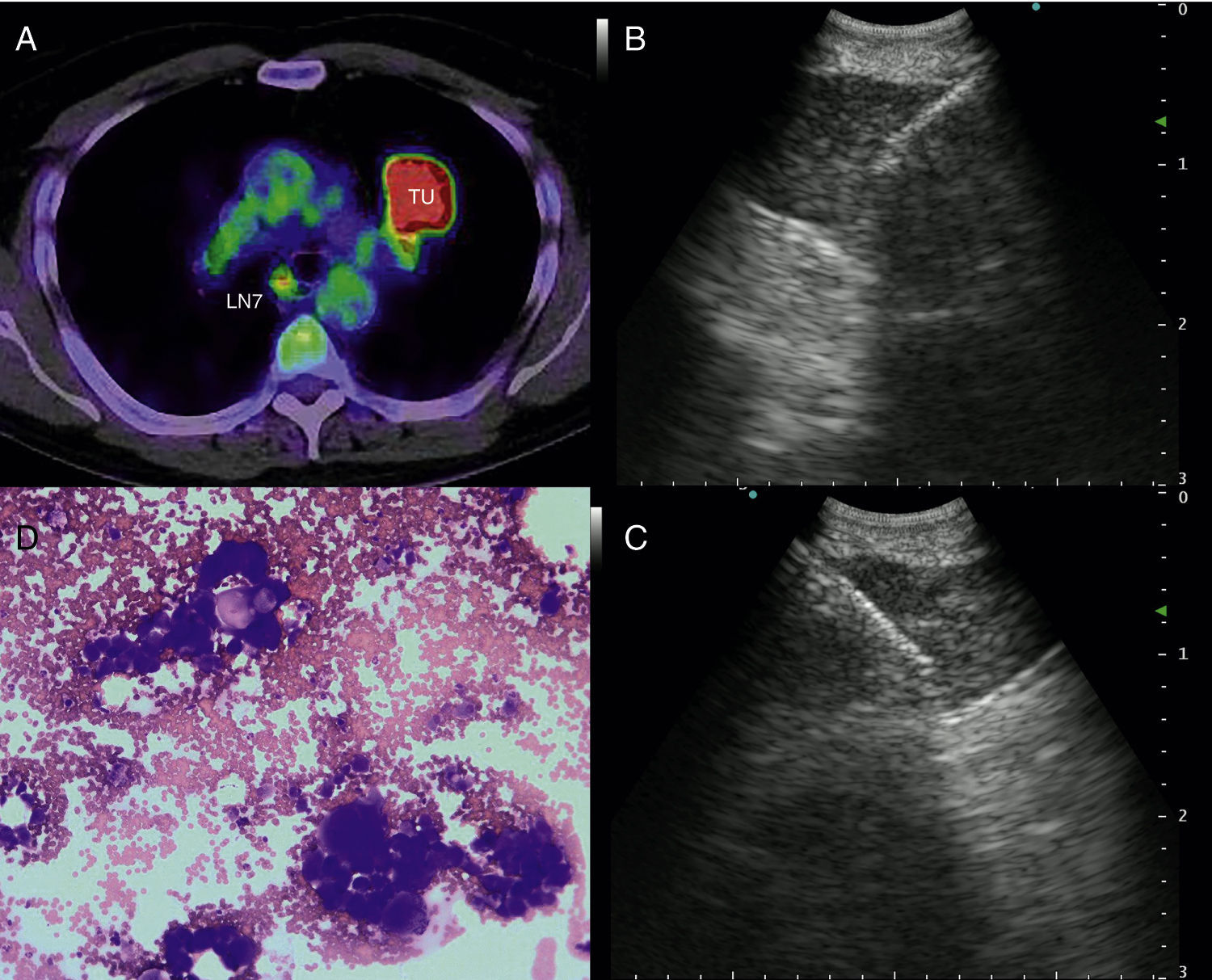
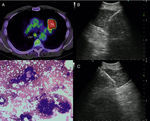
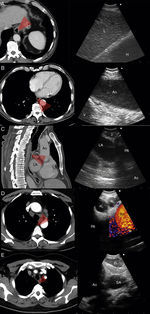
a) 56-year-old patient with a PET-positive pulmonary tumor in the left upper lobe suspicious of primary lung cancer (TU). In lymph node station 7 (LN7) there is increased FDG-uptake; (b) EBUS image of the lymph node in station 7; (c) corresponding EUS-B image of the same lymph node from esophagus with the same bronchoscope; (d) cytological image of the EUS-B-NA showing adenocarcinoma cells with background blood (courtesy of Cynthia van der Horst, MD, Glasgow, UK).
The accuracy of EUS-NA and EBUS-TBNA for evaluating mediastinal lymph node metastasis has been assessed in several clinical trials. In meta-analyses concerning this indication, the pooled sensitivity for EBUS-TBNA was 93%, whereas a sensitivity of 83% for EUS-NA was reported.13,14 Furthermore, in numerous studies it has been shown that the diagnostic value of a combined EBUS/EUS procedure was superior to either EBUS-TBNA or EUS-NA techniques alone, but no randomized controlled trials comparing these attempts had been performed yet 4.15,16
In a randomized controlled study, comparing immediate surgical staging to endoscopic staging, the combined endosonography approach with EBUS and EUS was superior to mediastinoscopy. The sensitivity for lymph node metastasis was 79% for surgical staging versus 85% for endosonography without subsequent surgical staging.17 Current international guidelines therefore recommend the endoscopic needle techniques over surgical staging as the first test for mediastinal staging in lung cancer patients.11,18,19
As previously mentioned, in two studies published simultaneously, both EBUS-TBNA and EUS-B-NA were performed in patients with suspected lung cancer for diagnosing and staging.4,5 The sensitivity for EBUS-TBNA was 91.5% and 84.4% respectively. Sensitivity for EUS-B-NA alone was 88.7% in one trial. For the combined approach of EBUS-TBNA plus EUS-B-NA the sensitivity rose to more than 90% in both trials. Importantly, the negative predictive value for malignancy using combination of EBUS and EUS-B was 96% in both trials. Particularly high negative predictive value for malignancy may in the future reduce the need for surgical confirmation, when the results of the needle acquired specimens are negative for malignant cells.
The utility of the EBUS-scope for performing EUS-B-NA in the same setting was confirmed in a further publication. In this prospective study, 123 patients with undiagnosed but suspected malignant lung lesions were assessed by both EBUS-TBNA and EUS-B-NA after at least one non-diagnostic conventional procedure.20 A definitive diagnosis was obtained in 87.6% of these patients and the diagnostic accuracy was 90.1%. In addition, the authors provided evidence that the combined use of the bronchoscope for EBUS-TBNA and EUS-NA enabled omission of surgical procedures and significantly reduced costs.
In a recently published meta-analysis, the overall diagnostic yield of EUS-B-NA combined with EBUS-TBNA was estimated.21 More than 1 000 subjects were included and the sensitivity of the combined procedure was significantly higher than for EBUS-TBNA alone (91% vs. 80%; p=0.004). The additional diagnostic gain of EUS-B-NA over EBUS-TBNA was 7.6%. The authors calculated that 10 combined procedures were needed to achieve one additional diagnosis, as compared with EBUS-TBNA alone.
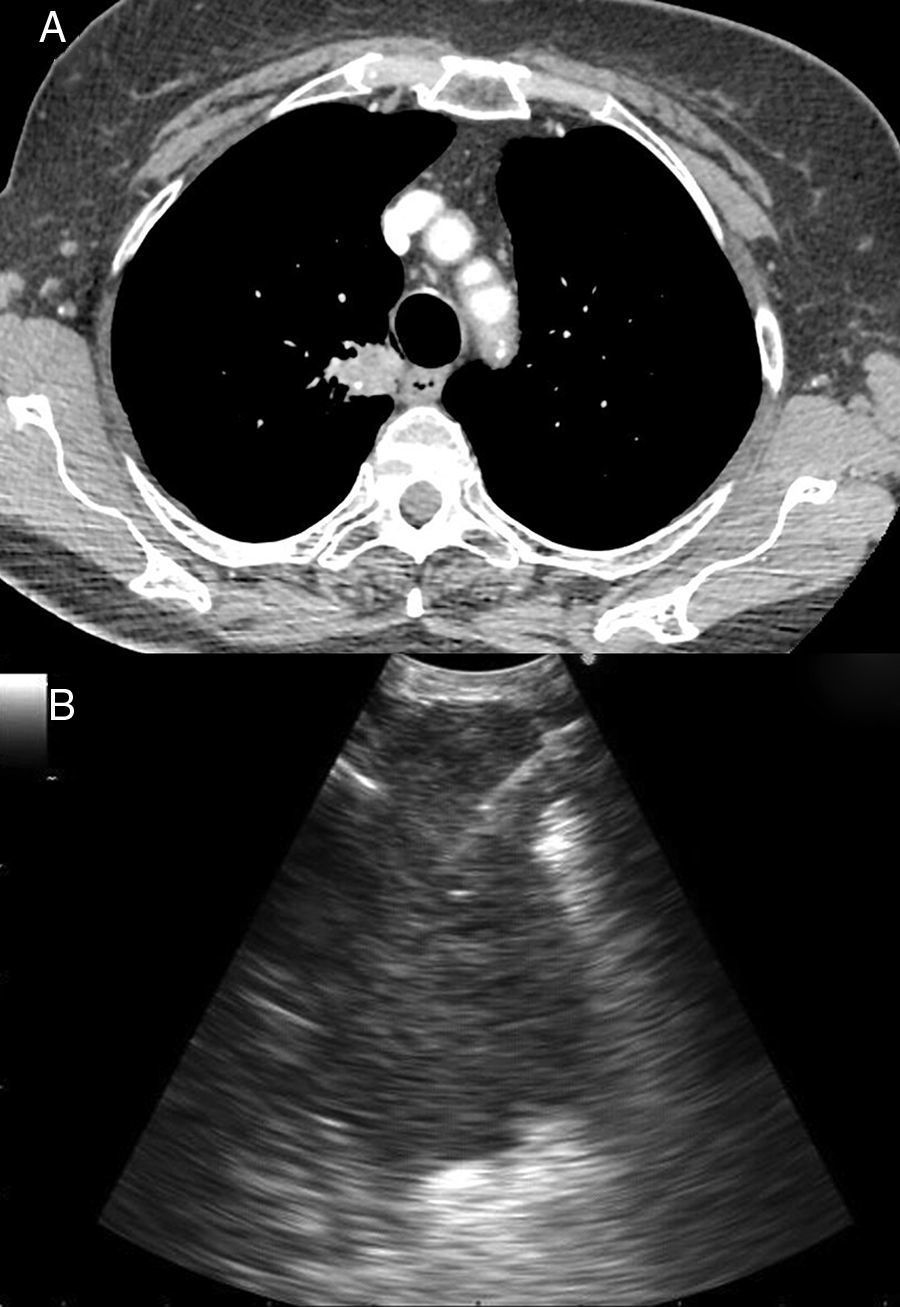
Other indications for EUS-B in malignancyIntrapulmonary tumors located adjacent to the mediastinal pleura are usually visible by endosonography. For that reason, EBUS as well as EUS are useful tools in diagnosing these pulmonary lesions in adults and children22–24 (Fig. 2). In a prospective trial, the feasibility of diagnosing intrapulmonary lesions by EUS-B-NA was demonstrated. In addition, the authors could confirm EGFR mutation and ALK fusion gene in some patients.25
Mediastinoscopy has been used to restage after neoadjuvant therapy and for the assessment of tumor recurrence after previous thoracic surgery. Still, this procedure is technically more difficult, less safe, and less useful for restaging. Besides, the evidence exists that EBUS-TBNA and EUS-NA can deliver comparable results.26,27 In a prospective study, 106 patients have been included after induction therapy for restaging.28 They were assessed by both EBUS and EUS using the same bronchoscope. In 286 biopsied lymph node stations, the total accuracy was 81.0% and the yield for the combined approach was higher compared with EBUS-TBNA or EUS-B-NA alone. In another publication, the authors confirmed loco-regional tumor recurrence by EUS-B-NA in cases where EBUS-TBNA was not possible.29
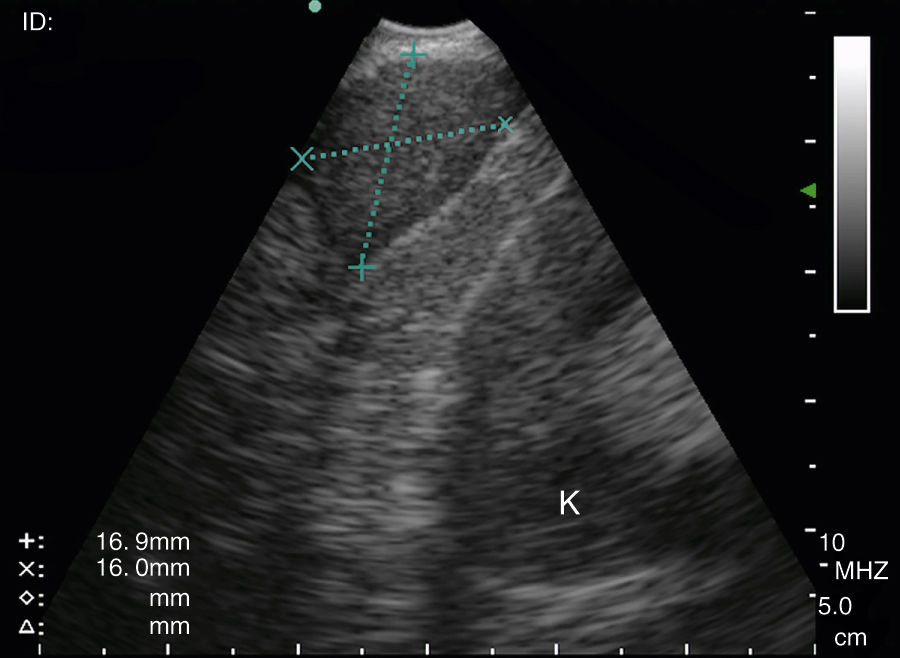
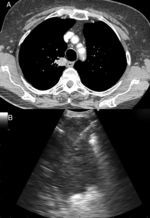
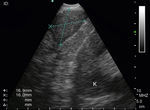
Puncturing the left adrenal gland (LAG) with an EUS-gastroscope is a well-established technique to confirm intra-abdominal metastasis.19,30,31 This is also possible with the EUS-B scope to assess and puncture the LAG endoscopically (Fig. 3). In a prospective multicenter trial, adequate tissue sampling was feasible in 93% with the EUS-scope and in 89% with EUS-B-NA in patients with suspected metastasis of the LAG.32 However, despite these encouraging reports, currently the use of the EBUS bronchoscope for sampling the LAG is not generally recommended.19
Finally, EUS-B may be used as an initial procedure for patients with suspected mediastinal lymphoma since it may decrease the need for more invasive approaches but sensitivity for final diagnosis and subtyping varies widely in different case-series.
Benign diseasesOnly a few studies were published using EUS-B in non-malignant diseases.
In clinical practice, most patients with suspected sarcoidosis present stage I or II disease, characterized by increased lymph nodes. Current diagnosis of pulmonary sarcoidosis is shifting from conventional flexible bronchoscopy with accessory techniques to other minimally invasive techniques, with improved efficacy and safety. In recent years, the detection of non-caseating granulomas in mediastinal lymph nodes was easily performed by EBUS-TBNA or EUS-NA.33,34 In a 2013, Oki et al. used EUS-B-NA in 33 patients to diagnosis stage I/II sarcoidosis.35 They achieved a diagnostic yield of 86% without complications.
In other retrospective studies, EUS-B-NA allowed the diagnosis of tuberculosis lymphadenitis in both children and adults, during the differential diagnosis of mediastinal or hilar lymphadenopathies.24,36 In these situations, the diagnostic yield increases if samples are sent simultaneously for microbiology, cytopathology, and mycobacterium tuberculosis-polymerase chain reaction.37
How to do EUS-B?Settings and equipmentThere are no specific recommendations for EUS-B regarding minimal room and equipment requirements hence they are extrapolated from conventional bronchoscopy and EBUS guidelines.
In most cases, EUS-B can be safely done as an outpatient procedure in a bronchoscopy unit. The suite should be properly equipped for patient monitoring. A secure intravenous access is mandatory and specific anesthetic drugs should be at hand. Oxygen delivery systems and resuscitation equipment should also be readily available.38 The procedure is usually performed under moderate or deep sedation to achieve appropriate patient comfort and collaboration.39 In some cases, depending on local settings, the goal of the procedure (systematic staging vs. targeted diagnostic puncture) and the operator's experience, general anesthesia may be a valid option.
A certified bronchoscopist with formal endosonography training, two nurses – one to assist during the procedure and other dedicated to sedation – and an anesthesiologist, in case of deep sedation or general anesthesia, should be the minimal personnel requirements to perform the procedure securely.
The presence of a cytopathologist for rapid on-site evaluation (ROSE) does not seem to affect the diagnostic yield, but reduces the number of punctures, procedure time and can be useful to evaluate the quality of material and quantity of malignant cells, namely when molecular testing is planned.39
In terms of equipment, different electronic linear scanning echoendoscopes are commercially available (Table 1). When deciding which one to choose, several factors need to be considered, including the existence of compatible equipment already available (e.g. video processors, ultrasonography console), characteristics of the EBUS scope (direction of view, flexibility, distal end size, diameter of the working channel, video quality and ultrasonic image), as well as costs and post-vending manufacture assistance. The final decision depends on a compromise between these options.
Differences between commercially available endobronchial ultrasounds.
| Company/model | Technology | Working channel (mm) | Direction of view (°) | Field of view (°) | Scanning area (°) | Angulation range (up/down°) | Distal tip diameter (mm) | Working length (mm) | Compatible ultrasound processors |
|---|---|---|---|---|---|---|---|---|---|
| Olympus BF-UC180F | Hybrid | 2.2 | 35 | 80 | 60 | 120/90 | 6.9 | 600 | EU-ME1, EU-ME2, EU-C60 (Olympus), Aloka Prosound Alpha 5, Alpha 7 and Alpha 10 (Hitachi) |
| Pentax EB-1970UK | Charged-couple device (CCD) | 2 | 45 | 100 | 75 | 120/90 | 7.4 | 600 | Hitachi Hi Vision Avius |
| Fujifilm Sonar EB-530US | Charged-couple device (CCD) | 2 | 10 | 120 | 65 | 130/90 | 6.7 | 610 | SU-1, SU-8000 |
Multiple disposable needles are available, all developed on the basis of the original construction of the Hancke-Vilmann needle system (GIP-Medizin Technik now Medi-Globe): a long-steel needle, a sheath and a handle for manipulation.40 Technological advancements have led to experimentation of new designs and materials that improve handling and performance but increase costs. Even though the main principles are the same, needles differ in several characteristics (Table 2). There are no prospective comparative studies showing the superiority of one needle39 over the others, so the operator needs to consider the handle design (ergonomic and maneuverability) and the characteristics of needle “per se” (durability, flexibility, echogenicity). Other features are, compatibility with the equipment and costs, especially if we aim for a needle that provides histological core samples (e.g. Vizishot Flex®, Echotip Procore®). According to recent guidelines, the operator should determine needle choice and either 21G or 22G are acceptable options for most cases.39
Differences between dedicated aspiration needles.
| Company | Model | Size (G) | Minimum working channel (mm) | Stylet tip | Compatibility |
|---|---|---|---|---|---|
| Olympus | Vizishot 1 | 21, 22 | 2 | Round | Olympus, Fujifilm |
| Vizishot 2 | 21, 22 | 2 | Round | ||
| Vizishot Flex | 19 | 2.2 | – | ||
| Boston Scientific | The Expect TM | 22, 25 | 2 | Round | Olympus, Pentax, Fujifilm |
| Cook Medical | Echotip | 22, 25 | 2 | Beveled | Olympus, Fujifilm (with adaptor), Pentax |
| Echotip Procore | 22 | 2 | Round | ||
| Echotip Procore | 25 | 2 | Beveled | ||
| Medi-Globe | Sonotip Pro | 22 | 2 | Round | Olympus, Pentax, Fujifilm |
| Sonotip Pro Flex | 22 | 2 | Round | ||
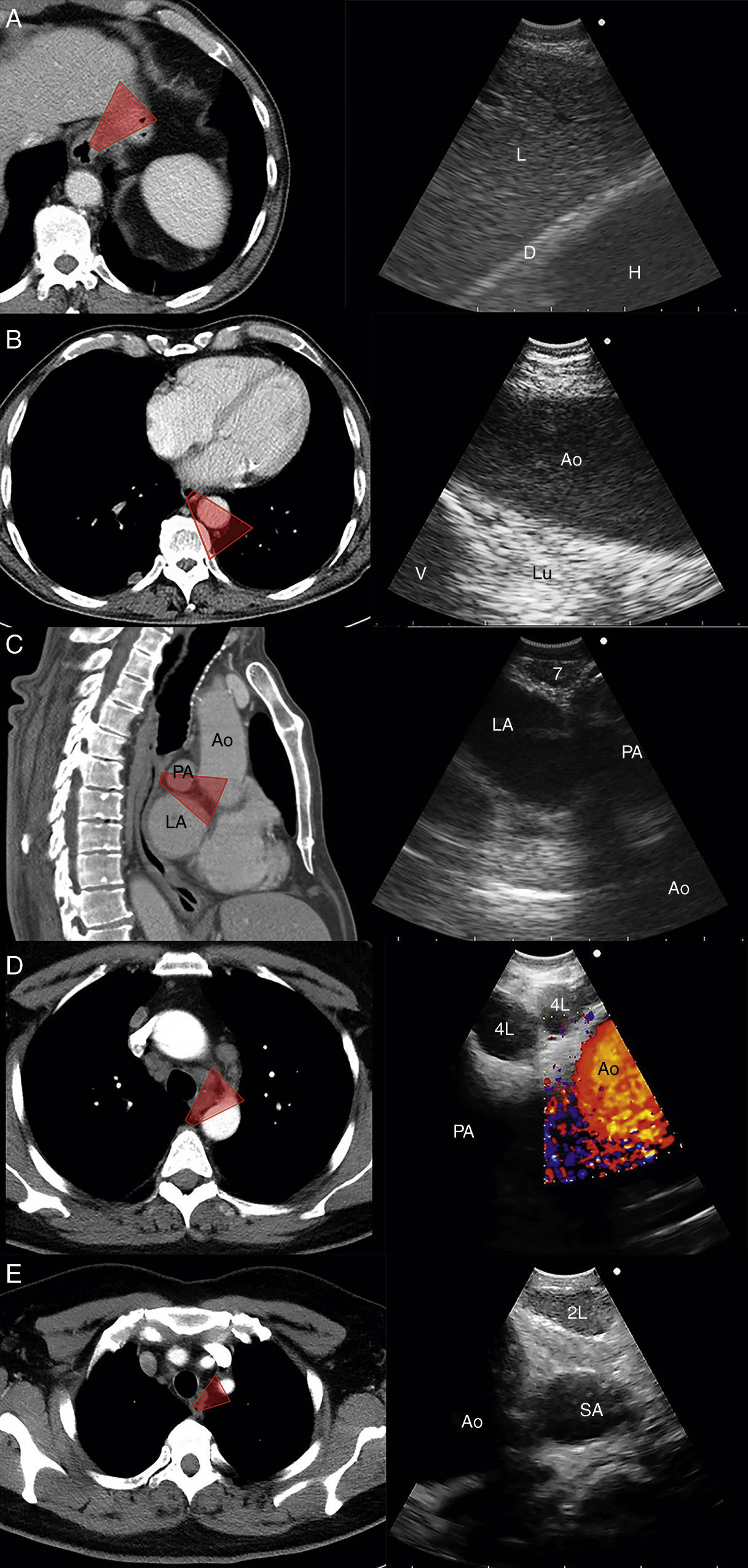
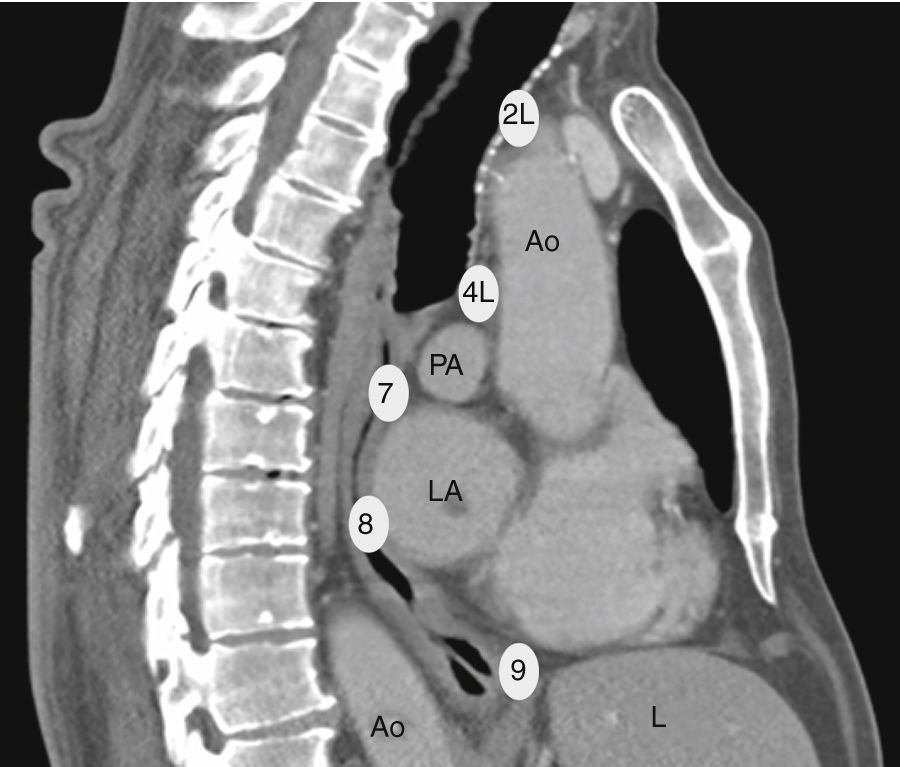
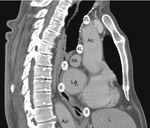
The EUS-B scope is inserted through the mouth into the esophagus gently rotating it with a screw movement, and advanced under visual control to the gastric fundus. While introducing the scope, suction should be used only in an intermittent manner, to avoid deflating the esophageal tract completely. The endoscopic view is not normally useful and endosonography scanning guides the procedure. From this moment on, continuous suction is mandatory to facilitate the contact between the ultrasound transducer and the esophageal wall. At the cardia region, the transducer is turned back until the abdominal aorta is clearly visualized in its longitudinal diameter (Fig. 4). To identify the LAG, the tip of the bronchoscope is slid down the aorta, up to the celiac trunk and mesenteric artery, which are the first vessels extending from the abdominal aorta. At this point, the EUS-B scope is turned counterclockwise (if the physician is standing behind a patient lying in supinal position this movement turns the transducer to the left) to visualize the left kidney, the spleen, and the LAG just behind the celiac trunk and mesenteric artery. After seeing the LAG, the transducer is brought back to its standard neutral position at the cardia. In front, the left lobe of the liver, diaphragm and heart are clearly visualized (Fig. 4A), and to the right the hepatic veins can be followed as they approach the inferior vena cava. Then scanning proceeds by withdrawing the transducer to the distal esophagus. The EUS-B scope is turned around from the descending aorta and back to the aorta again (Fig. 4B), visualizing retroperitoneal and para-aortic lymph nodes. If the scope is turned slightly more to the left, the diaphragm is clearly seen on the left corner and the right atrium on the right. At this level, the left pulmonary ligament lymph nodes (station 9) can be detected. During further withdrawal of the scope, from the distal to the middle part of the esophagus, para-esophageal (station 8) and lower para-aortic lymph nodes are seen posteriorly. Then the heart is visualized anteriorly, with the left ventricle coming first into focus, at that point the atrioventricular valves and the left atrium with pulmonary vein close to the esophageal wall. While withdrawing the scope slightly more, subcarinal lymph nodes (station 7), especially the posterior group, are clearly visible (Fig. 4C), and to the right, reflections from the trachea and the proximal wall of the right main bronchus are seen, as well as the right pulmonary artery in a transverse section. Further retreating the scope and looking anteriorly at the tracheal reflections at its lower part, slightly to the right, the posterior part of the right paratracheal lymph nodes (station 4R), behind azygos vein, can be found in many cases. At this level, rotating the EUS-B scope to the left will permit to detect the lower paratracheal (station 4L) and deeper aorto-pulmonary window lymph nodes (station 5) between the pulmonary artery and aorta (“mickey mouse effect”) (Fig. 4D). On the left, the entire descending intrathoracic aorta can be followed along the esophagus, and para-aortic lymph nodes (station 6) may be visualized through the aortic arch. Behind the aorta, reflections from the thoracic spine are well seen. As the EUS-B scope is further withdrawn, the left subclavian artery and upper left paratracheal lymph nodes (station 2L) are identified on the left side, and just behind them the left internal jugular vein is often found (Fig. 4E). To the level of the upper part of the esophagus, both lobes of the thyroid gland can be seen and below them seldom mediastinal highest lymph nodes (station 1) can be seen.
The technique for needle biopsy aspiration is similar to the one performed for EBUS-TBNA, but often easier because of the softness of the esophageal wall. On the other hand, the EUS-B-NA seems to be more difficult because of incorrect adjustment to the esophageal wall. Therefore, the reach of EUS-B is the same as for EUS: it is possible to puncture the LAG, left lobe of the liver, lymph node stations 2L, 4L, 7, 8, 9 (Fig. 5) and in specific situations the lower part of station 5, posterior part of station 4R and station 1.
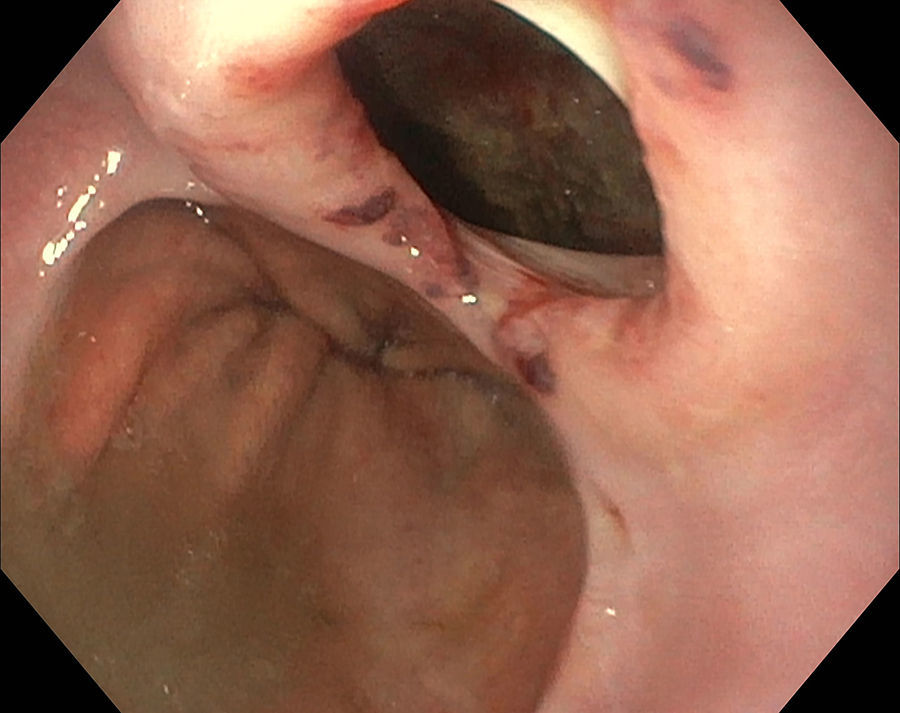
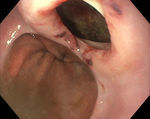
SafetyThe intra-esophageal introduction of an EBUS-scope and its use for EUS-B-NA is safe. No serious complications have been reported. Nevertheless, the development of a lymph node abscess after simultaneous EBUS-TBNA was described in one subject.13 In a systematic review of combined EUS-NA and EBUS-TBNA for staging of mediastinal lymph nodes in lung cancer, severe complications were reported in two patients (0.3%), the patient with the lymph node abscess and a pneumothorax in an additional patient.41 However, the EBUS bronchoscope is not only thinner, but also sharper at the tip. This fact needs to be considered when the bronchoscope is used in the esophagus. If the esophagus is narrowed or a diverticulum is present, but not easily visible due to the side-view optic, there is a significant risk of iatrogenic bleeding due to mucosal damage or even of perforation of the esophageal wall (Fig. 6).
Training and competencyEndoscopy is part of the European respiratory curriculum as well as the integration of knowledge regarding thoracic radiology, mediastinal anatomy, and lymph node map.42
It makes sense to train individual bronchoscopists in EBUS and EUS-B at the same time and enable them to perform complete endoscopic staging in one session 2.43 However, even the criteria for competency in EBUS are unclear and left to subjective judgment of trainers. The arbitrarily defined number of supervised procedures does not guarantee that the trainee will master the procedure in the end, since every individual follows his own learning curve.44 Hence the focus should be directed toward evaluation of the individual's performance and outcomes by predefined criteria.45 New trainees in EBUS/EUS-B-NA should follow a structured training curriculum consisting of simulation-based training followed by supervised practice on patients.2,42,46 Self-learning is no longer an acceptable method since it inflicts distress, causes potentially false therapeutic decisions, and should be therefore discouraged.47
The next important issue is maintaining competency after initial EBUS/EUS-B training. Diagnostic yield can improve with continuous practice and the number of complications tends to decline.48,49 Furthermore, there is evidence that dedicated interventional pulmonologists are more likely to perform procedures properly than general pulmonologists who do not perform them on the regular basis.50
Future of EUS-BEUS-B is a relatively recent method that for which a wider implementation and further development are expected. So far we only have reports from centers of expertise, which are promising and assure EUS-B potential.4,5,32 Therefore, we perceive the safe implementation of EUS-B by previously trained EBUS professionals in clinical practice as the first step in the future of EUS-B-NA development. It remains open how this can be executed but focused additional training with collaboration of EUS specialists is perhaps the logical solution. Additionally, data from further trials should be published, to gain more evidence about feasibility, diagnostic yield, and complications.
Further equipment development is heading toward thinner and more flexible EBUS scopes, with extended range of visibility and biopsy possibilities in the lungs.51 The same instruments need to be re-evaluated in the upper gastro-intestinal tract to judge whether their performance matches the recent EBUS-bronchoscopes.
EBUS and EUS-NA offer the most accurate diagnosis of metastatic lymph node infiltration, but the accuracy depends on the appropriate selection of the lymph node and targeting the suspicious area within the node.52 The European guidelines for elastography suggest that EUS-elastography adds information to the B-mode evaluation of lymph nodes and can accurately guide needle biopsy in the stiffer part of a suspicious lesion.53 EUS-B elastography was feasible and reproducible in parallel with the EBUS elastography, when the same lymph nodes were evaluated.54 Perhaps, such data would be even more interesting in the LAG, which currently remains for future evaluation.
ConclusionIt took longer for chest physicians to start performing EUS-B compared to EBUS and not all institutions do the procedure on a routine basis.
The use of EUS-B-NA for cytological diagnosis of malignant and benign diseases is feasible and safe. The results of EUS-B-NA are comparable to just EUS-NA and a combined approach with EBUS-TBNA is recommended for lung cancer staging since it increases diagnostic yield and accuracy. Chest physicians should execute EUS-B after proper structured training. New indications, scientific data and equipment developments are bound to happen over the coming years.
Conflicts of interestThe authors have no conflicts of interest to declare.