With the purpose of establishing a consensus around clinical orientations for professionals involved in managing patients with sleep breathing disorders (SBD), an interdisciplinary group of scientific societies involved in this field discussed and reviewed all the published international guidelines from the American Dental Association, American Academy of Sleep Medicine, American Academy of Dental Sleep Medicine and the European counterparts. Treatment of SBD is multidisciplinary and should be made in concert with the patient, the sleep physician, and the qualified dentist to solve the individual, social, and economic burden of the disease,. This consensus document represents the current thinking of a team of Portuguese experts on managing patients with SBD based on the available evidence.
Snoring and obstructive sleep apnea (OSA) are common in clinical practice. Snoring is the sound caused by the vibration of soft tissues of the upper airway and has an extremely high prevalence in the general population, estimated to be around 40 % in adult men and 20 % in adult women.1 However, snoring is not only a sound problem; several studies associate it with damaging effects, namely atherosclerosis,2,3 and with nervous lesions on the upper airway muscles.4,5 Roncopathy is often associated with OSA, and an objective measurement of snoring severity constitutes a strong predictor for concomitant OSA after adjusting for specific risk factors.6
OSA is a sleep-breathing disorder that involves complete or partial cessation of breathing while sleeping due to complete or partial pharyngeal obstruction.7–9 If OSA is associated with frequent arousal during sleep, excessive daytime sleepiness and other symptoms, it is termed OSA syndrome.8
Epidemiological data suggests that, globally, 936 million adults between 30 and 69 years have mild to severe OSA and 425 million have moderate to severe OSA.10 The prevalence of OSA in the United States adult population is estimated to be around 12 %, with nearly 80 % of patients undiagnosed, which represents a massive economic impact.11,12 Data from the HypnoLaus study from Switzerland, showed a prevalence of moderate-to-severe sleep-disordered breathing of 23.4 % (95 % CI 20.9–26.0) in women and 49.7 % (46.6–52.8) in men.13 When considering the prevalence of sleep apnea syndrome, i.e., OSA associated with symptoms, the prevalence is considerably lower (<5 % in all groups).13 A 2019 literature-based analysis estimated a 17.0 % prevalence of mild OSA and 12.5 % of moderate to severe OSA in Portugal.10
Among all the sleep breathing disorders (SBD), OSA presents a high prevalence and cardiovascular and metabolic impact,14 although simple snoring can also have a negative health impact, such as an increased risk for cardiovascular disease or social impact, due to poor quality of life for bed partners.7,9
OSA and associated comorbidities share many similar pathophysiological mechanisms. As they exist together, mortality risk in OSA increases largely with the increased number and severity of comorbidities, and can lead to serious health conditions, such as systemic hypertension, coronary artery disease, stroke, atrial fibrillation, and congestive heart failure. They can be also responsible for increased working and motor vehicle accidents, with an overall decreased quality of life.7,9,15,16
Continuous positive airway pressure (CPAP) is taken as gold standard therapy for moderate to severe OSA syndrome, i.e. symptomatic OSA, but other options are available, depending on the case severity, patient compliance, and other factors subject to medical evaluation, such as oral appliance therapy (OAT) with intraoral devices (OA) - custom-made and titrable - and surgery.7,17,18
OAs are indicated for patients with mild to moderate OSA, with no comorbidities and primary snoring, and are accepted as an alternative therapy for patients with severe OSA who do not respond to or are unable or unwilling to tolerate positive airway pressure (PAP) therapies.19
The most effective OA are mandibular advancement devices (MAD) that stabilize the lower jaw in a forward and downward position, maintaining airway patency during sleep.7,19 MAD were shown to decrease the frequency and/or duration of apneas, hypopneas, respiratory effort-related arousals (RERA), and/or snoring events, as well as to improve nocturnal oxygenation.19 They also reduce daytime sleepiness and improve quality of life measures in OSA, with a better adherence comparing to CPAP.7,20
There are some contraindications for MAD that include severe periodontal disease, severe pre-existing temporomandibular disorders (TMD), lack of adequate retention (inadequate dentition or implants), and severe gag reflex. Poor dexterity or hand function can also compromise device handling.21 Despite these contraindications, depending on the status of the implants and adjoining tissues, MAD can be used with implant patients without edentulous dental arches, or in unrehabilitated completely edentulous patients, considering the upper dental arch.
Dilator muscles of the upper airway play a critical role in maintaining an open airway during sleep.22 Therefore, exercises that target the oral cavity and oropharyngeal structures are being explored to treat OSA. Myofunctional therapy (MT) and correct tongue positioning in the oral cavity have been described to improve mandibular growth, nasal breathing, and facial appearance.22
Policies on SBD management and the specific roles of dentists and physicians in the process are changing due to recent findings. In 2018, American Dental Association (ADA) recognized standards for screening, treating, and managing adults with SBD, describing bidirectional referral patterns, in which the qualified dentist should refer to the physician, and the physician should refer to the qualified dentist.23 The importance of collaboration between professionals and the interdisciplinary needed was emphasized.
This consensus clarifies the role of qualified dentists as an essential member of the multidisciplinary team in screening, treating, and managing patients with SBD, according to the consensus between Portuguese scientific societies operating in this field. This article aims to recommend a patient-focused protocol, finding agreement between relevant institutions, and national and international guidelines.
Material and methodsThe Portuguese Society of Pulmonology (SPP), Portuguese Society of Stomatology and Dental Medicine (SPEMD), and Portuguese Society of Temporomandibular Disorder, Orofacial Pain and Sleep (SPDOF), as well as a representative member of the Portuguese Dental Association (OMD), discussed and reviewed published international guidelines from ADA, American Academy of Sleep Medicine (AASM), American Academy of Dental Sleep Medicine (AADSM)7,18,24 and European counterparts.17
The commission was composed of both board-certified sleep medicine specialists and experts with proficiency in the use of OA, particularly MAD in adults with OSA. The members presented and discussed relevant findings and guidelines regarding the clinical management of SBD, in the most recent SPP 2021 Autumn Meeting.
The goals of the task force were: (1) to simplify the screening, treating, and managing protocol of patients with SBD, finding consensus in the reviewed evidence; (2) to clarify the role of the qualified dentist in the defined protocols; (3) to discuss the economic costs for treating SBD patients, discussing the relevance of new governmental policies.
ResultsThe role of dentistsAccording to the 2021 Oral Health Barometer, released by the Portuguese Dental Association, it is estimated that 61 % of Portuguese visit a dental doctor at least once a year for professional oral hygiene procedures or other therapies,17,25 in accordance with the published data. These conditions set dentists in an ideal position to perform OSA screening.
A medical dentist can participate in screening, treating, and managing adults with SBD7,17,18,23,26 and can provide a more streamlined and cost-effective model of care.18,23 Special training or experience is required to deliver informed care. Dentists should be educated according to the AADSM-defined requirements to be a “Qualified Dentist” in dental sleep medicine.7 AADSM advocates that dentists should have a valid state license and proof of liability coverage and possess additional training or experience in this area of care.7 Dentists should use objective data according to their scope of practice and as defined by their state dental practice acts.27
In Portugal, the competence in dental sleep medicine was framed in December 2021 in the Regulation Document for Sectorial Competences.28 However, the specific criteria to be recognized as a dentist qualified in dental sleep medicine in Portugal are not well defined yet.
ScreeningWhen faced with a patient with snoring, the dentist must investigate whether it is isolated snoring or associated with other symptoms that suggest sleep-related respiratory pathology.19,23,29 Dentists should screen patients for SBD, starting with the partner's perception of both sleep and awake symptoms (snoring, witnessed apneas, gasping, sleepiness) and by evaluating the upper airway.23,26 Clinical history should be oriented to seek OSA risk factors, like the presence of excess weight, smoking habits, alcohol drinking history, sedative drugs, and family history of OSA.30 Finally, questionnaires should be applied like the Berlin Questionnaire, the STOP-BANG score to assess OSA probability, and the Epworth Sleepiness Scale (ESS)23 to assess daytime somnolence.19,23,26
The prevalence of OSA is higher in specific populations, namely the obese, people diagnosed with congestive heart failure, atrial fibrillation, hypertension, type 2 diabetes, pulmonary hypertension, or who had a stroke; therefore, it is fundamental to evaluate the clinical history.
There are certain professionals who, due to the nature of their work, are more at risk of the effects of OSA in sleep privation, namely people whose work involves heavy driving, like professional truck drivers, airline pilots, and heavy machinery operators.31
Physical examThe qualified dentist should carry out a physical examination of the identified at-risk patients in the screening phase. Baselines should be recorded for each patient to monitor future changes. Features to be evaluated that may suggest the presence of OSA include increased neck circumference (>43 cm in men, >40 cm in women), body mass index (BMI) >30 kg/m2, a Modified Mallampati score of 3 or 4, lateral peritonsillar narrowing, macroglossia, tonsillar hypertrophy, elongated/enlarged uvula, high arched/narrow hard palate and nasal abnormalities.24 The presence of retrognathia and/or overjet should be noticed23.In some cases, the patient should be referred for an ear, nose, and throat evaluation.23 When OA can be indicated, it should be noticed that the number of teeth included in the appliance framework can compromise the retention and efficacy of the device or even the loss of teeth in the future.23 Also, the location and morphological integrity of teeth and periodontal health should be accessed to orientate OA selection. TMD and orofacial pain should be screened. Occlusal analysis and intraoral and extraoral photographs are mandatory and recommended to access any future variations. A retrognathic mandible, shorter soft palate, and low positioning of hyoid bone have all been associated with favorable outcomes. However, these associations are weak and, again, cannot be relied on for clinical decision-making.21 A cephalometric evaluation is not always required for patients who will use an OA, although it is recognized that appropriately trained professionals should perform this examination.24 This evaluation is considered as an option, and not as a guideline, for physical examination.32
SBD diagnosisSBD diagnosis should always be made in a multidisciplinary team including the qualified dentist, the sleep unit and the sleep doctor, as recommended by the international guidelines. As for screening the role of dentists, family doctors, pulmonologists and ear–nose–throat (ENT) doctors as well as others healthcare professionals that compose the multidisciplinary seep team including the qualified dentist, the sleep unit and the sleep doctor, as recommended by the international guidelines.24
Overnight polysomnography (PSG) or a home sleep apnea test (HSAT) should be prescribed, aimed at collecting objective data to determine if the patient suffers from an SBD. HSAT portable monitors may be indicated for the diagnosis of OSA in patients for whom in-laboratory PSG is not available or possible due to immobility, safety, or critical illness.32 Sleep tests can be divided into four types:32
Type 1: in laboratory full attended PSG (≥ 7 channels) in a laboratory setting
Type 2: ambulatory full unattended PSG (≥ 7 channels)
Type 3 (HSAT): limited channel devices (usually using 4–7 channels)
Type 4: 1 or 2 channels usually using oximetry as one of the parameters
A PSG (type 1 or 2) uses a 7 channel minimum recording system with an electroencephalogram (EEG), electrooculogram (EOG), chin electromyogram, airflow, oxygen saturation, respiratory effort, and electrocardiogram (ECG) or heart rate.24 A type 3 HSAT includes, at a minimum, the record of airflow, respiratory effort, and blood oxygenation. The type of biosensors used to monitor these parameters for in-laboratory PSG are also recommended to use in type 3 HSAT and include an oronasal thermal sensor to detect apnea, a nasal pressure transducer to measure hypopneas, oximetry, and, if possible, calibrated or uncalibrated inductance plethysmography for respiratory effort.24
PSG can provide the Apnea Hypopnea Index (AHI) and respiratory disturbance index (RDI). AHI can be fixed as the number of apnea and hypopneas per hour of sleep.7 RDI measures the number of apnea, hypopneas and RERA.33 The severity of OSA can be determined using the AHI: mild OSA (5≤AHI<15/h), moderate OSA (15≤AHI<30/h) and severe OSA (AHI≥30/h).24 However, other surrogate parameters like hypoxic burden, hypoxia load, obstruction severity, or phenotypes based on symptoms and comorbidities, should be considered together with AHI in the disease management and treatment decision-making processes.34,35
PSG is routinely indicated for the diagnosis of sleep disorders, while type 3 HSAT should be used as part of a comprehensive sleep evaluation in patients with a high pre-test likelihood of moderate to severe OSA. However, they are not indicated in patients with major comorbid conditions, for instance, cardiorespiratory disease, neuromuscular condition, suspected hypoventilation, chronic opioid medication use, stroke or insomnia.24 When PSG is not available or possible (immobility, safety or critical illness), HSATs can also be used for OSA diagnosis and to monitor response to non-CPAP therapies.24
Even though AASM and AMA have previously published recommendations stating that HSAT should be ordered by a physician,23,26 nowadays it is considered that the qualified dentist also has the training and education necessary to order this exam.18,36 The diagnosis of medical diseases and verification of treatment efficacy remains the responsibility of the sleep physician, as the PSG prescription.18,36
Type 4 tests are not considered a diagnostic exam for OSA.
Treatment optionsSleep-disordered breathing tends to get worse and not to cure spontaneously.16 The treatment option for moderate to severe symptomatic OSA (OSA syndrome) is CPAP therapy, considered the gold-standard treatment.18,37 This therapy should be used considering OSA risk factors and, therefore regarding behavioral and positional therapies.37,38
Behavioral treatment options include weight loss, ideally to a BMI of 25 kg/m2 or less; weight reduction surgery in selected cases; physical exercise; positional therapy when indicated; and avoidance of alcohol and sedatives before bedtime.24,37 The patient should be aware that weight reduction in obese patients with OSA is associated with a trend of improvement in breathing pattern, quality of sleep and daytime sleepiness, and that is recommended to reduce these important risk factors.16,24
Supine position can also affect airway size and patency with a decrease in the area of the upper airway, particularly in the lateral dimension.24 Positional therapy can be initiated by positioning devices (e.g., alarm, pillow, backpack, tennis ball) and keeping the patient in a non-supine position, with better patency, which can improve AHI.32,37
In selected adult patients, hypoglossal nerve stimulation and myofunctional therapy can be considered for specific cases seeking alternative treatments, although both constitute conditional recommendations.37
Videotapes, handouts, websites, and brochures can be used as resources for patient education.24 Medical treatment of ENT diseases with pharmacological treatment should also be considered.
For specific cases, other treatment options include surgery, such as maxillofacial surgery (maxillo-mandibular advancement) or otolaryngologic surgery.23,26,39
Treatment options should always be discussed by a multidisciplinary team including the qualified dentist, the sleep unit and the sleep doctor.24
OA treatmentThe candidates for treatment with OA are adult patients who request treatment of primary snoring (without obstructive sleep apnea) or patients with mild to moderate OSA with no comorbidities. OA is an accepted alternative therapy for patients with severe symptomatic OSA who are intolerant to CPAP therapy or request an alternative therapy.7,19,29 In fact, adherence with OA is better in patients with OSA.20 Oral appliances reduce the AHI, the arousal index, daytime sleepiness as well as to improve quality of life measures in adult patients with OSA.3 It also improve nocturnal oxygenation (oxygen desaturation index and minimal oxygen saturation).7,20,40
Although CPAP is superior to OA in terms of normalizing respiratory parameters, AHI, oxygen desaturation index and minimal oxygen saturation37,40–42 CPAP and OA demonstrate comparable effects in symptoms and health-related quality of life measures. We found in multiple RCTs and meta-analyses similar improvements on daytime sleepiness, general physical and mental health, driving simulation assessment and nocturia.41,43–45
The ideal candidate for OA treatmentYounger age, lower BMI, and smaller neck circumference have been related to successful treatment, as well as the female gender. Evaluation of nasal patency and function should also be done to reduce the risk of non-responders. Low AHI indicated by PSG and position-dependent OSA have been proposed as predictors of success. As described above, other factors can favor or compromise the outcomes, namely, the number of teeth included in the appliance framework the characteristics of the mandible, the palate, and the hyoid bone, and non-anatomical traits such as a sensitive respiratory control system and a low arousal threshold.23,46,47 However, none of these factors is essential for successful treatment, which can be achieved in overweight patients and those with more severe disease.21
To orientate OA selection, the teeth location and morphological integrity should be accessed as should the periodontal health. To access any future variations, it is mandatory and recommended to perform an occlusal analysis and intraoral and extraoral photographs.
More recent research has focused on titrating mandibular advancement during sleep studies to assess the potential efficacy and determine optimal mandibular protrusion. Early prototypal studies have been promising, but these methods are resource-expensive and seem unlikely to be widely adopted.21
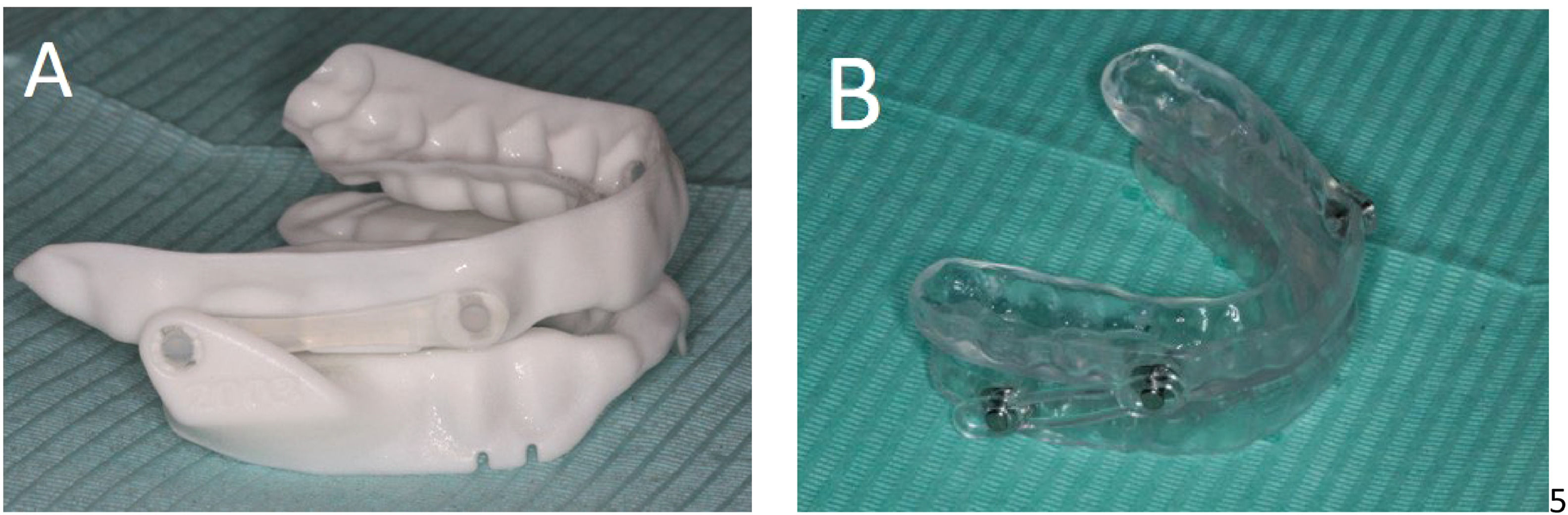
Intraoral devicesAn effective OA should be individualized and titratable, made of biocompatible materials and engage both the maxillary and mandibular arches (Fig. 1).19,23 It should maintain a stable retentive relationship to the teeth, implants or edentulous ridge and retain the prescribed setting during use.19 The OA should allow the mandible to be advanced in increments of 1 mm or less with a protrusive adjustment range of at least 5 mm and have the possibility of reversing the advancement.19
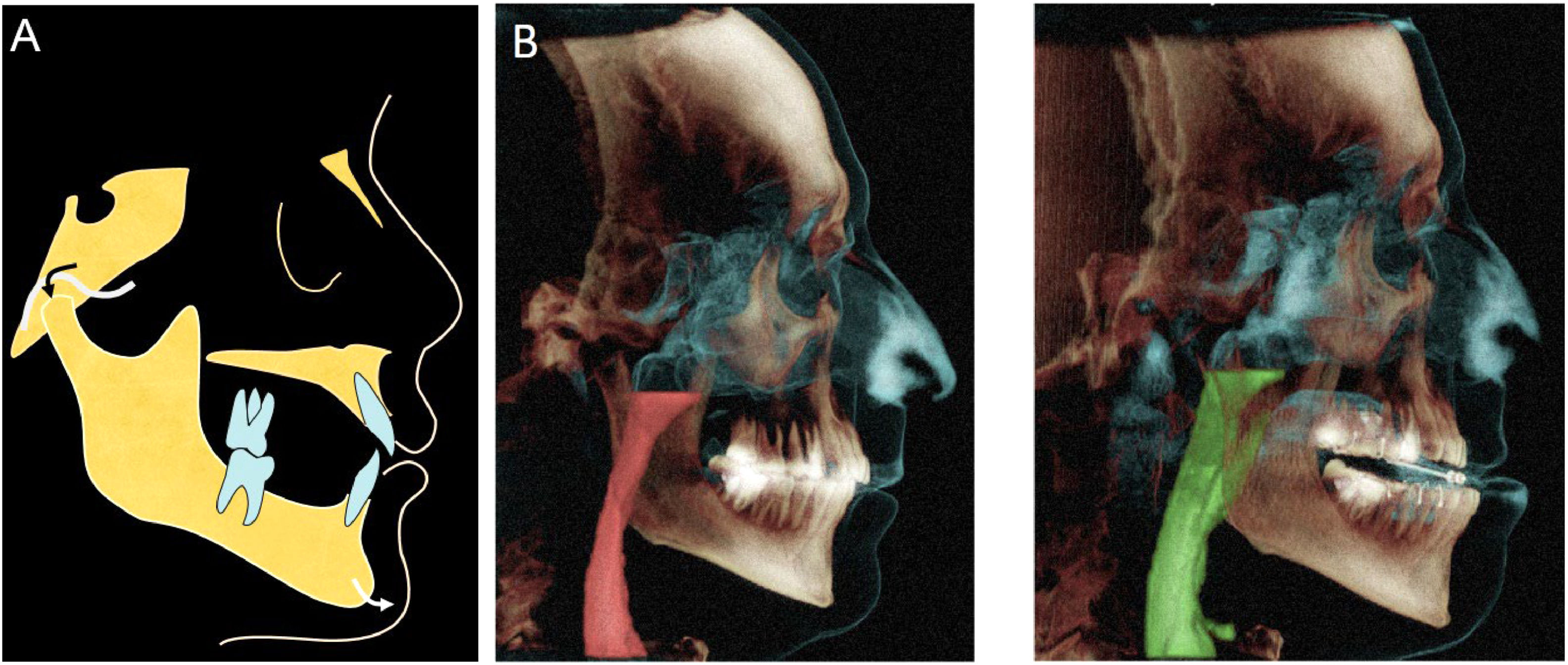
Qualified dentists with appropriate training in the field should provide the devices.18,23,26 An ideal device should have anatomical evidence that supports maintaining airway patency during sleep (Fig. 2).19
Schematic diagram of the working mechanism of a mandibular advance dispositive (MAD) (A) advancement and forward positioning of the mandible during sleep. (B) Corresponding upper airway volumes in relation to the anatomical structures without (left panel) and with (right panel) mandibular advancement.
Studies employing sleep endoscopy enabled direct observation of a patent airway upon mandibular advancement in sleeping patients who respond favorably to oral appliance therapy.19
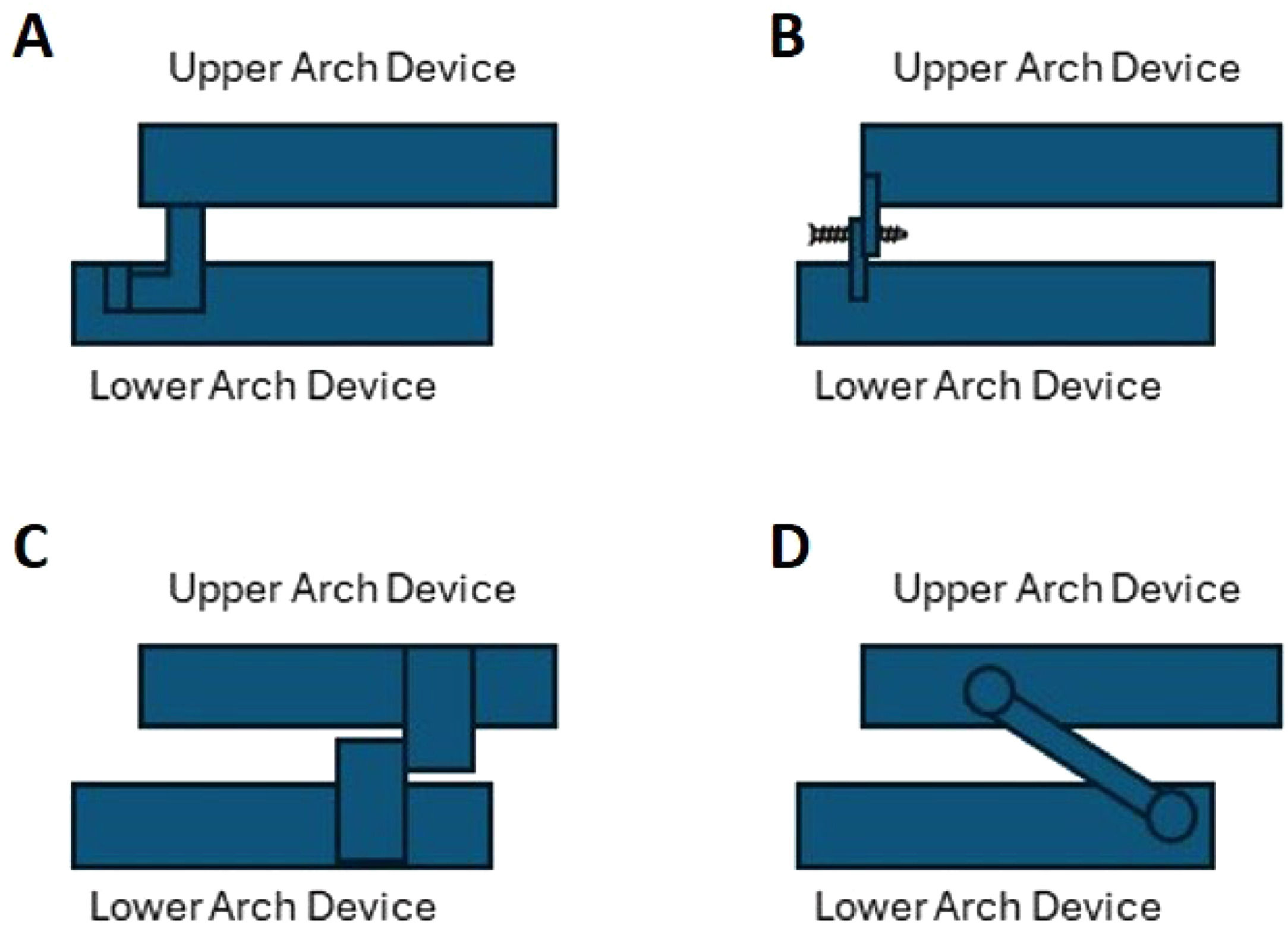
There are several OAs in the market, with different features, being MAD the most accepted by the scientific academies. A custom-made OA is fabricated using impressions or a scanner of mouth and bite from an individual patient's oral structures; in contrast, non-custom OAs are primarily pre-fabricated, also known as “boil and bite devices”.7 OAs can also be classified as titratable or non-titratable.7 During the titration procedure, which is only adjustable during treatment in titratable devices,7 the mandible is gradually positioned and stabilized in a more anterior position to achieve a maximum therapeutic effect on opening the upper airway.33 The concept of a custom-made MAD has evolved from the ‘mono-bloc’ type of device where upper and lower parts are rigidly connected towards the current ‘duo-bloc’ types or titratable MAD. The rigid mono-bloc MAD can restrict mandibular movements, sometimes producing discomfort, and there is evidence suggesting significant improvements with ‘dual-block’ when compared with ‘mono-block devices’, namely the possibility of titling, which is an unequivocal advantage (Fig. 3).48,49
Schematic overview of titratable, duo-bloc MAD designs used in current clinical practice: (A) MAD with an anteriorly articulating component that allows the MAD for adjustment of the appliance; (B) MAD with attachments for adjustment of mandibular protrusion in the frontal teeth area; (C) MAD with two lateral positioning attachments that permit incremental protrusion of the mandible; (D) MAD with lateral telescopic rods that force the mandible into an anterior position.
The qualified dentist will need to determine an appropriate endpoint for the MAD advancement, usually between 50 % to 80 % of the patient's maximum protrusive range.21 A dose dependency seems to be a frequent finding in the literature, although a larger protrusion is not always associated with a corresponding reduction in AHI.33
Smaller increments of advancement can allow the evaluation of subjective parameters while minimizing potential temporomandibular discomfort.19 Progressive mandibular advancement performed during the clinical titration of the dispositive was shown to decrease AHI and oxygen desaturations,19 and the ability to titrate protrusion according to efficacy and tolerance is the key advantage of these devices.21
In the absence of an optimal titration strategy many possible titration protocols have tried to achieve this target protrusive position.33
Titration can be multiparametric, combining the evolution of subjective symptoms and objective measurements of the severity of SBD. Objective parameters can be obtained by using a single-channel measurement (generally measuring nasal airflow or oximetry) with a portable monitoring device type 4, a portable monitoring device type 3 (monitoring ventilation or airflow, heart rate or electrocardiogram and oxygen saturation), or full polysomnography (7 channel). Since no laboratory facility or night-time technician is needed, a major cost of traditional polysomnography is removed. Portable monitoring device type 3 is in general an easy and reproducible tool to assess the evolution of SBD in response to treatment.33
There is no consensus in the literature regarding the definition of a successful treatment outcome, or which combination of objective and subjective criteria should be used to determine if a titration protocol is successful or not.33 For patients with OSA, the desired outcome of treatment includes the resolution of the clinical signs and symptoms of OSA, and the normalization of the apnea-hypopnea index and oxyhemoglobin saturation.29
Considering the vertical adjustability of MAD, several studies suggested that increased vertical dimension decreased patient acceptance and had no consistent impact on efficacy.19 In the absence of valid research data, one should keep the vertical opening and dimension to a minimum during the gradual titration of the mandibular protrusion.10
OA effectiveness evaluationOutcome indicators to monitor therapy include evaluation of the resolution of sleepiness, patient quality of life and satisfaction assessment, adherence evaluation, and evaluation of presence of protective and absence of worsening risk factors.18
As titrating MAD during sleep studies is expensive,21 titration is still determined with “trial and error”.33 This reinforces the importance of a final sleep test equal to the initial diagnosis (always interpreted by the sleep physician), to allow comparison with baseline indicators.18,36 Some authors support that a type 3 test, including polysomnography or an attended cardiorespiratory sleep study, should be performed to ensure satisfactory therapeutic benefit from OAs, with the oral appliance in place after final adjustments of fit are made.18,29 If the treatment is sub-therapeutic, the physician and dentist should collaborate to discuss calibration or other treatment possibilities - for instance, combining CPAP therapy with the treatment with OA.50
Follow upAn annual follow-up performed by the sleep specialist is required regarding sleep apnea treatment, i.e. suppression of breathing disturbances and improvement of quality of life. The sleep specialist should regularly evaluate the breathing disturbances with PSG and HSAT. Additionally, regular evaluation of the dental and oral cavity situation should be performed by the dentist.37
Those on chronic therapy (CPAP, OA, positional therapy) should have regular follow-ups, and those with the elimination of OSA (weight loss, surgery) should be monitored for continued risk factor modification and to look for the eventual return of symptoms.24
Follow-up protocol after the final calibration should include a patient evaluation every six months for the first year and at least annually thereafter.50 Patients diagnosed with primary snoring may be treated without objective follow-up data; however, they should be re-evaluated at least annually.50 Follow-up sleep testing is not indicated for patients with primary snoring.29
Besides evaluating patients’ requirements for treatment with oral appliances, the qualified dentist should also proceed with the necessary follow-ups.17If the patient's annual assessment reveals symptoms of worsening OSA or the potential need for additional adjustments to the device, the dentist shall communicate this information to the patient's physician.50
Most complications of MAD therapy are mild and temporary. Short-term side effects of OAs utilization in the stomatognathic system usually occur during the adaptation period in the first few weeks of therapy. They can include hypersalivation, dry mouth, damage to teeth or restorations, dental pain, gingival irritation, myofascial pain and TMD discomfort.21,23
Adherence to OAs declines over time, mostly due to appliance intolerance and TMD issues.29 TMD disorders can be a true obstacle to using these appliances, although they are usually transient, and any pain appears to decrease in intensity with continued use.21 During the patient adaptation, mandibular exercises may improve discomfort and allow continuity of treatment; this can be softened with a physiotherapy professional.21
Long-term dental side effects are also identifiable, namely decreases in overbite and overjet, proclination of the lower incisors, retroclination of upper incisors and occlusal alterations in posterior teeth.21 Appropriate planning and monitoring may avoid these dental effects, and the benefits are assumed to compensate for the inconvenient effects.21 Additionally, bite changes can influence the maintenance or alteration on the degree of advancement of the device and this situation should be controlled in patients’ follow-ups.51
If the device is lost or broken, a new comprehensive evaluation should be completed to enable replacement.23 In case of relapse or worsening of initial signs and symptoms, weight gain and changes in overall health, the patient should also be examined by a qualified dentist.
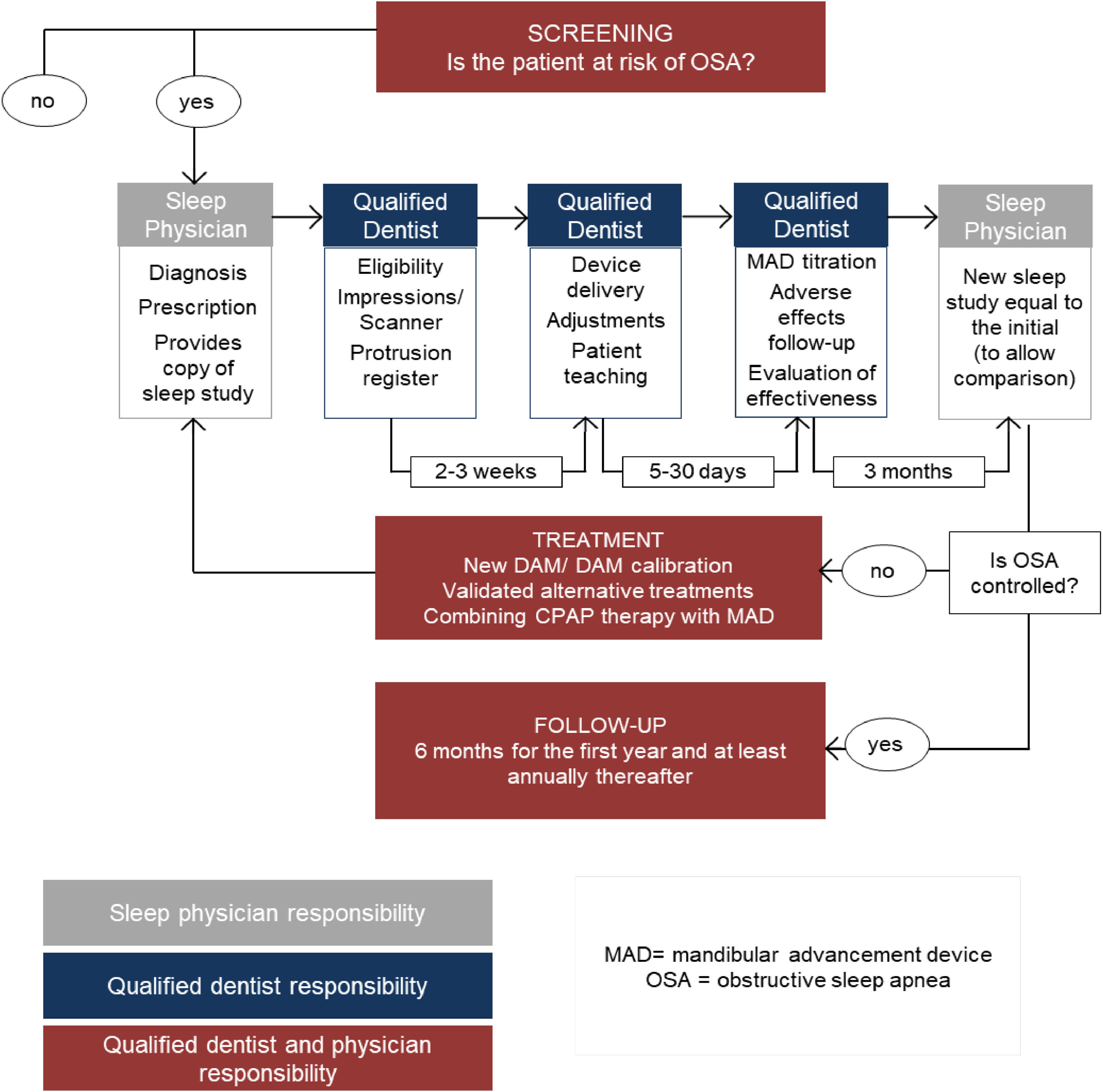
Flowchart between multidisciplinary teams - an integrative modelFig. 4 presents a proposed flowchart of OSA screening, treatment, and follow-up between multidisciplinary teams.
Bidirectional referral patterns are recognized, with the qualified dentist referring to the physician and the physician referring to the qualified dentist, always sharing relevant clinical information.18,23,26
The dentist must have a role in screening patients with snoring or OSA and establishing diagnosis together with a sleep physician.17,18 The main purpose of SBD risk patient management protocols is to establish the communication flow between the multidisciplinary team; qualified dentists should screen sleep apnea but diagnosis and treatment efficacy should be verified by physicians, even when the screening, the OA selection and the positioning and consequent follow-ups are made by the qualified dentist.18
DiscussionEarly detection and treatment of SBD benefit a patient's health and quality of life.8 Symptoms such as snoring, which should be taken seriously, are still widely ignored, leading to a high prevalence of undiagnosed sleep apnea.23,30,52
CPAP therapy is highly effective in OSA syndrome but can have low adherence due to treatment intolerance and poor benefits in mild disease.21 Therefore, OAs executed by qualified dentists are a validated option for these patients. Therapy with OAs should be performed exclusively by dentists who have received specific training in sleep medicine/dentistry and the use of these devices.7 Simple physical examination of anatomic indicators, such as increased neck circumference, increased body mass index, Modified Mallampati Score and anatomic abnormalities of the oral cavity, allows the identification of the patient at risk.23 Most recent international guidelines assure that the qualified dentist has ideal conditions to screen SBD in the population as they usually see the patients for routine appointments to observe the mouth and surrounding anatomic structures.17,36
Interdisciplinary cooperation and a multimodal approach are essential for screening, treating, and managing patients with SBD. The key to a patient-focused treatment is a consensus between the roles every different professional must play.
Although the proposed approach only identifies patients with high-pretest probability, the main point is that the physician or qualified dentist does the screening, so there can be an increase in the possibility of diagnosing OSA in a dental appointment. The approach here proposed includes clinical assessment, probability questionnaires and referral to the sleep unit to carry out a sleep study. Despite the low probability of patients with typical symptoms, this would be a way of finding at least those. In the future we expect a more thorough screening process to be made using Type 4-Screening sleep studies for all patients suffering from some kind of continued sleep alteration, including patients with different symptoms or relevant co-morbidities but with less clear symptoms. Still, OAs execution, including titration of the appliance to maximize the results and reduce side effects, and follow-up of OA therapy is undertaken by the qualified dentist. Treatment of OSA patients, within the limits and scope of the dental profession, as management of related pathologies such as dry mouth, sleep bruxism, orofacial pain and related headaches, is also a qualified dentist's responsibility.17,53 Nevertheless, diagnosis of SDB, usually through PSG test, and the final follow-up for treatment evaluation, must be performed only by the sleep physician.7,17,18
PSG is routinely indicated for the diagnosis of sleep-related breathing disorders. HSAT tests can be used in patients without significant comorbid medical conditions and when PSG is not available or possible.32
Titration protocols that use a titratable OA during sleep to predetermine an effective protrusive position may be valuable,33 such as type 2 and 4 tests with HSATs. However, since titrating MAD during sleep studies is expensive, titration still is determined by a “trial and error” approach with type 3 tests.21,33 A final sleep type 1 or 2 test for baseline results comparison, equal to the one performed at initial diagnosis (always interpreted by the sleep physician), is recommended.18,36
OSA has a high rate of underdiagnoses, estimated to be around 80 %.11,12 Direct costs of OSA are associated with comorbidities such as hypertension, coronary disease, stroke, dysrhythmias, diabetes, and the risk of motor vehicle or workplace accidents.10–12 Indirect economic costs also represent a burden to the healthcare system and include decreased productivity at work, reduced quality of life, and stress on interpersonal relationships.10–12 Among employees being actively treated, a 73 % reduction in preventable driving accidents was identified.11,12 The economic burden of SBD in Portugal is not well established yet, but it is assumed that they will have a massive impact, as it happens in the US, where this burden is estimated to be billions of dollars.11,12
The healthcare system should focus efforts on preventing and early diagnosing SBD, instead of focusing on acute problem solving, which has a much higher economic and social cost, even costing lives.10–12
This paper reinforces the need to invest in training general dentists to track patients suspected of SBD and refer them to sleep physicians or sleep-qualified dentists to confirm OSA diagnosis. Also, it is necessary to value the specialization of dentists in the sleep specialty, reinforcing its role in multidisciplinary teams for the treatment of sleep disorders and to continue discussing these issues among the professionals involved in SBD management, promoting research concerning the existing knowledge gaps for which more evidence is needed.
ConclusionsIn conclusion, oral appliances, are recommended in adults with primary snoring (without obstructive sleep apnea) or patients with mild to moderate OAS with no comorbidities; MAD should be used in CPAP intolerant patients rather than no therapy; MAD being custom and titratable, are the most effective OA; titration protocols that use a titratable OA during sleep to predetermine an effective protrusive position may be valuable; and dentists can order sleep tests to improve, confirm or follow-up treatment efficacy of OA. Identifying signs and symptoms of risk, done in routine appointments, places the qualified dentist in ideal conditions to screen SBD patients, combating the high rate of underdiagnoses, which represents a massive social and economic impact. Nevertheless, dentists should refer the patient to a sleep physician to confirm the diagnosis, determine treatment success, and follow-up on general health. SBD is a complex problem that requires multidisciplinary management. Resolution depends on the performance of the patient, the sleep physician, and the qualified dentist to understand the problem and access all the available treatment options. The healthcare system should focus efforts on preventing and diagnosing SBD early and minimize the consequences of its economic and social burden.