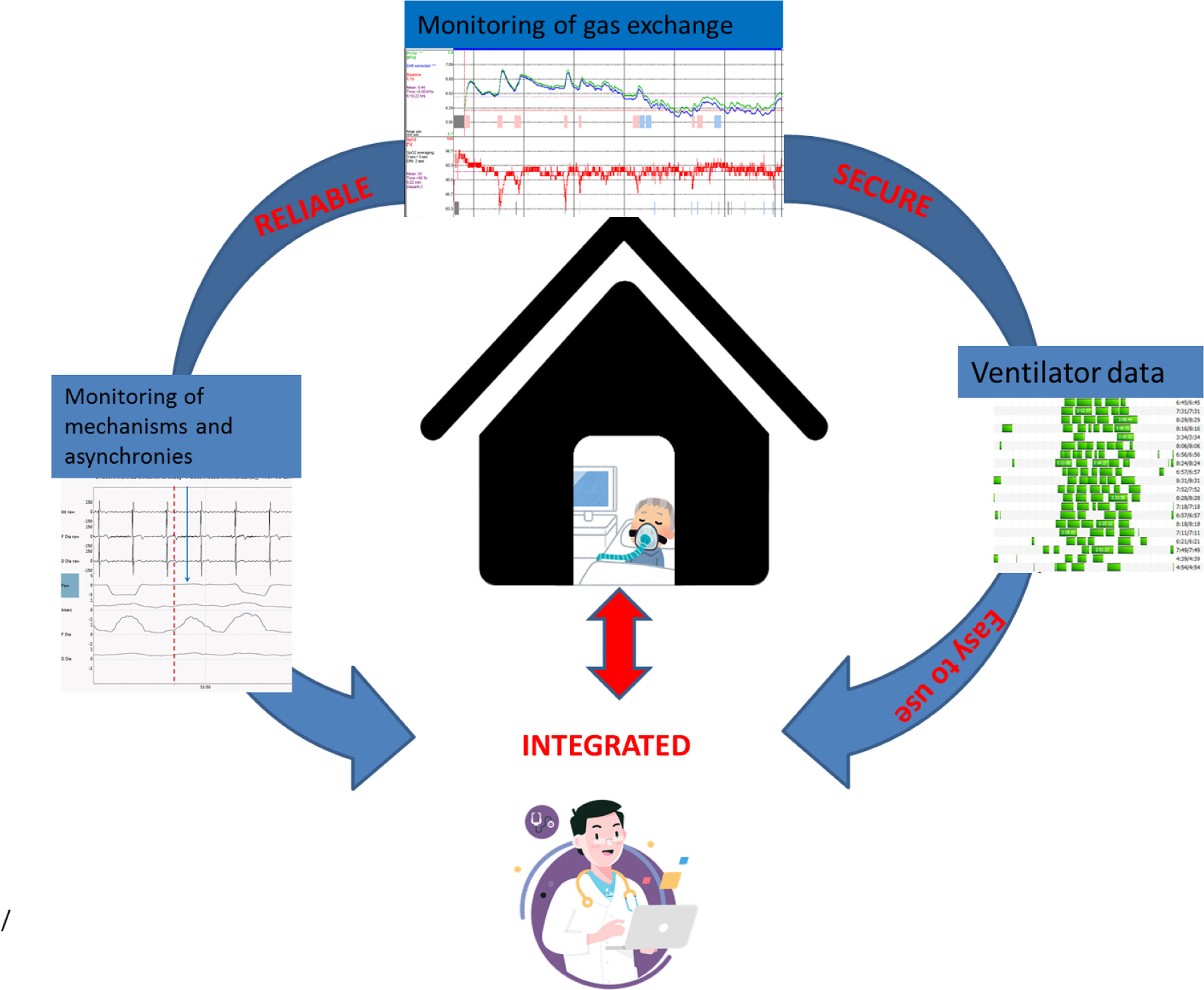
In this part of the review series “Tricks and tips for home mechanical ventilation”, we will discuss the evidence with regard to the place and manner of home mechanical ventilation initiation and follow-up. Outsourcing more and more of this chronic care to the home situation is a big challenge for the future: especially for the home situation, monitoring has to be non-invasive, reliable and easy to use, data security needs to be ensured, signals need to be integrated and preferably automatically processed and algorithms need to be developed based on clinically relevant outcomes.
In the Netherlands, home mechanical ventilation (HMV) was founded 60 years ago.1 The first patient transferred home while remaining dependent on mechanical ventilation in 1960 was a patient who survived poliomyelitis anterior acuta.2 Home non-invasive mechanical ventilation (NIV) has emerged since the mid-1980s for patients with chronic respiratory failure. In the past decades, indications have evolved and the number of patients on HMV has increased. Recent data from Switzerland have shown an increase in home NIV prevalence from 15.1 to 37.9 per 100.000 with an increase in especially chronic obstructive pulmonary disease (COPD) patients and patients with Obesity Hypoventilation Syndrome (OHS).3 Worth noting is the very variable practice between countries, both with regard to numbers as well as underlying diseases.4 Nowadays, most patients on home mechanical ventilation are ventilated non-invasively.4 With an increase in patients with pre-existing chronic respiratory failure to be initiated electively on HMV, the place of HMV set-up has changed from set-up exclusively in the ICU to nowadays an increasing tendency for home set-up.
The key to successful therapy is a careful set-up and titration of ventilatory support. Hospital admission was for a long time considered necessary to set-up HMV. Also, in some countries, patients are admitted to hospital to titrate HMV during follow-up.5 However, with increasing patient numbers and limited health care resources usually needed for acute care services, hospital admission for elective HMV initiation and titration has become less justifiable, if it is clear that other home-based protocols are at least non-inferior. In this review we will discuss the evidence with regard to the place and manner of HMV initiation and follow-up. Outsourcing more and more of this chronic care to the home situation is a big challenge for the future: especially for the home situation, monitoring has to be non-invasive, reliable and easy to use, data security needs to be ensured, signals need to be integrated and preferably automatically processed; algorithms need to be developed based on clinically relevant outcomes (Fig. 1).
Before HMV is startedIrrespective of the place of set-up, once HMV is started, therapeutic goals should be clear and communicated with the patient. A thorough analysis of the patient’s motivation, goals, home situation including available support of family members, medical history and current medical situation is warranted. While HMV is usually titrated on reduction in arterial carbon dioxide (PaCO2) levels, as a surrogate marker of ventilatory efficacy, patient wishes usually extend beyond gas exchange improvement. Independently of the underlying disease, patients suffer from symptoms of nocturnal hypoventilation, such as sleeping badly, frequent awakenings, morning headaches, tiredness and loss of vigilance.6 On the other hand, expectations of HMV are different in various underlying diseases. While for patients with thoracic restrictive diseases (TRD) and particularly more slowly progressive neuromuscular diseases (NMD), such as M. Duchenne, the initiation of HMV has been shown to increase live expectancy dramatically,6 for patients with rapidly progressive NMD such as amyotrophic lateral sclerosis (ALS) and COPD, improvement of symptoms and health-related quality of life (HRQoL) might be the most important goal, as these are progressive diseases and improvement in survival has been shown less convincingly.
Furthermore, a thorough analysis of comorbidities is needed. Especially in patients with concomitant cardiac failure, caution is needed as high ventilatory pressures might increase intrathoracic pressure, might reduce right ventricle preload and thus cardiac output. However, the effect of mechanical ventilation on cardiac functioning is a complex interplay between potential negative and positive effects, depends on the patient’s underlying condition, the applied ventilatory settings and compensatory mechanisms.7,8 Therefore, the exact net effect is unpredictable9 and it is advisable to monitor these patients more carefully during HMV set-up (for example by continuous or repeated blood pressure measurements).
Where to startIn many countries, HMV is still initiated in the hospital,5 albeit there is no consensus on exactly how and where it should be organized: the places where it is done (i.e. pulmonary ward, respiratory care unit, intensive care unit) vary considerably, as do the costs. With increasing prevalence of HMV, this will place a huge burden on the health care system. Furthermore, patients with severe disability in daily living often prefer to stay at home where they have organized their care instead of being transferred to a rather stressful hospital environment.
In the last decade, several trials have emerged showing that home initiation of NIV in patients with neuromuscular diseases, restrictive thoracic disease and COPD is non-inferior to in-hospital initiation with a reduction of >50% of the costs.10–13 It has to be noted that strict remote monitoring of ventilator data and gas exchange and daily remote or direct “live” support during the initiation period was offered in these trials. The recently presented results from the OPIP multicenter trial showed that in Obesity-Hypoventilation patients, NIV initiation at home with an auto-adjust mode without further monitoring and support was not cost-effective as patients initiated at home had far more healthcare contacts afterwards compared to patients initiated by a nurse-led overnight NIV titration in hospital.14,15
In daily practice, the strict protocol of home monitoring and daily remote assistance that was used in the recent positive trials, might not be possible in all centers, depending on the structure of the healthcare system and HMV team, reassurance possibilities for home care and local distances from the center to the patient. Secondly, the success of changes to remote HMV are dependent on not only technical possibilities but also on technical reliability and ease of use. Thirdly, privacy issues of data transfer need to be secured. Extending the ability to monitor at home with a reliable, solid, but easy to use independent home telemonitoring module or in-built ventilator module with telemonitoring capabilities would be a significant improvement in the care of today’s and future patients on chronic NIV. Some ventilator manufacturers are developing these integrated modules for transcutaneous gas exchange monitoring, although it general use is limited by the fact that each manufacturer has developed his own software and platforms, and thus users (caregivers and patients) have to adapt to these differences when switching to a different ventilator.
What to monitor (at home and in the hospital)Gas exchangeThere is no consensus on how to initiate and titrate long-term home ventilation. To assure effective nocturnal ventilation, at least gas exchange should be monitored. Monitoring of daytime gas exchange as a reflection of nocturnal gas exchange may be useful. However, it has been shown that with a daytime PaCO2 <6.0 kPa (<45 mmHg), up to 26% of the cases of (milder) nocturnal hypoventilation might be missed.16 Also, in patients with a limited ventilatory capacity, daytime PaCO2 may rise again after patients are disconnected from their ventilator. This thus does not reflect insufficient ventilatory support per se but merely reflects a too limited capacity to sustain benefits during the day. For these reasons, to judge ventilatory support, it is preferable to monitor also nocturnal gas exchange.
Pulse oximetry is a simple, easy way to detect oxygen desaturation. However, the specificity of the detection of nocturnal desaturation as a marker of nocturnal respiratory events is low and not reliable when patients use supplemental oxygen.17 Nocturnal carbon dioxide measurements are therefore needed to monitor alveolar ventilation. The ‘gold standard’ to measure this is to retrieve repeated samples of arterial blood via an arterial line. However, arterial cannulation is uncomfortable, expensive and demands continuous monitoring in the hospital by trained personnel, in most hospitals only available in high care units. Early morning sampling of PaCO2 is also less appropriate, since this is always after arousal and a period of spontaneous awake breathing. Capillary blood gas analysis is an alternative for arterial blood gases, but is still invasive, not appropriate for home monitoring and cannot be measured continuously during the night. Also, for capillary measurements it is known that arterial oxygen levels are underestimated when considering capillary levels compared to gold standard arterial levels, especially in hypoxemic patients,18 with fingertip samples showing even more deterioration compared to ear lobe sampling.19
A noninvasive way to assess PCO2 continuously is by measuring peak expired carbon dioxide tension (PetCO2) or transcutaneous carbon dioxide tension (PtcCO2). An advantage of continuous monitoring is that trends and thus also the effect of mechanical ventilation can be directly observed. While measuring PetCO2 is not a reliable measurement for PaCO2 in patients with a huge amount of dead space ventilation, such as in COPD,20 PtcCO2 values are comparable to arterial (gold standard) values, and can be used for the purpose of alveolar ventilation monitoring.21,22 Therefore, we suggest using nocturnal PtcCO2 to monitor HI-NIV gas exchange goals, both during the initiation period as well as during patient follow-up.
Ventilator data monitoringMany ventilatory devices contain sensors and built-in software that provide information about compliance, settings, and estimated values of tidal volume, leaks, breathing frequency, minute ventilation, percentage of breaths triggered by the patient, and the apnea-hypopnea index (AHI) over an extended period. These parameters can help identify abnormal nocturnal events, and in some cases, the causes of these events. However, important concerns have been raised regarding variables recorded by ventilatory devices: 1) are they reliable and 2) are they clinically relevant, i.e. does monitoring of these variables lead to improved outcomes?23
Objective data on hours of ventilator use provide important information. On the one hand, a threshold numbers of hours of daily use is probably necessary to obtain clinical benefits.24 A recent study in a large group of patients on HMV showed that less than 4 h usage per day was associated with worse survival.25 Furthermore, interrupted patterns of ventilation or an overall decreased use may indicate inappropriate settings, adverse effects or patient discomfort. On the other hand, increasing use over time may also predict deterioration.26,27 Thus daily use monitoring from ventilator hardware is reliable and seems to be of clinical use.
Data on tidal volume, leaks, breathing frequency and apnea/hypopnea index (AHI) are estimated parameters and when interpreting these data one should be aware of the drawbacks. First, reliability of these measures is limited. Tidal volume estimates are influenced by leaks, as with a single limb circuit with expiratory leak port—which is almost always used with NIV—expiratory volumes cannot be measured. Most noninvasive ventilators tend to underestimate the tidal volume delivered, especially with high IPAP levels and significant leaks.28 Furthermore, the clinical relevance of measuring these parameters has not been thoroughly investigated. The question is whether data depicted by the ventilator relate to ventilatory efficacy with regard to improvement in gas exchange, improvement in health-related quality of Life (HRQoL) and symptoms and patient comfort.
In conclusion, compliance data should be used especially during follow-up of patients using HI-NIV. Other data provided by the ventilator may be of value, however, the usefulness, reliability and validity of most parameters require further evaluation.
Extended monitoringIn some difficult to ventilate patients, more extended monitoring might be necessary, ranging from performing additional poly(somno)graphies to quantify sleep quality and nocturnal sleep related events to sophisticated methods to quantify patient-ventilator (a)synchrony, lung mechanics and patient effort.
Poly(somno)graphyWhen initiating long-term HMV, a sleep study can be considered a) before initiation to detect (concomitant) sleep-related breathing disorders (apnea’s/hypopnea’s), b) during initiation to adjust ventilator settings and c) during follow-up in patients in whom goals are not met.
Before starting a patient with chronic respiratory failure on long-term HMV, a poly(somno)graphy might be useful in patients in whom obstructive or central events or other sleep related problems are suspected. Especially in patients with OHS, concomitant obstructive sleep apnea (OSA) may direct the choice of therapy to continuous positive airway pressure (CPAP) instead of bilevel positive airway pressure (BiPAP), as studies have shown that both short- and long-term outcomes are comparable.29–31 However, also in other patient groups, such as COPD,32 Myotonic Dystrophy33 but also Amyotrophic Lateral Sclerosis,34 obstructive and central sleep related events are prevalent. In those patients, if chronic respiratory failure is accompanied by concomitant sleep apnea, it is unknown which mode (CPAP or BiPAP) is preferred. In practice, this will depend on the severity of both, symptoms and patient preferences and goals.
During initiation of chronic ventilatory support, in some hospitals, polysomnography (PSG) is used as standard manner to adjust ventilatory settings.35,36 Although with PSG, sleep quality, respiratory events, patient-ventilator asynchrony, can be detected, PSG is expensive, complex and not available in all centers/settings. Furthermore, at least in COPD/OHS, it has been shown that PSG-adjusted NIV does not lead to more improvement in gas exchange compared to nurse led titration based on ventilator data and transcutaneous measurements of gas exchange.37 Despite these findings, we hypothesize that PSG might have a role in the titration and follow-up of HMV in patients who are difficult to initiate on NIV, in whom concomitant sleep related disturbances are noticed and in patients in whom goals are not met. Further research is needed to detect those parameters/disturbances that affect clinical outcomes and automatic algorithms based on those parameters which can be used to change ventilatory settings.
Patient-ventilator (a)synchrony, lung mechanics and patient effortMonitoring and trying to adjust for patient-ventilatory asynchrony (PVA) is very common with acute mechanical ventilation in the intensive care unit.38 However, with long-term home NIV, its value is still unknown, also because description and quantification of PVA is not standardized. Monitoring PVA helps to identify abnormal respiratory asynchronous events during the night. However, monitoring PVA is complex and it is controversial whether in long-term HMV this leads to improved clinical outcomes. A recent pilot proof-of-concept clinical trial showed a trend toward greater improvements in daytime PaCO2, HRQoL and sleep quality when using simple gas exchange monitoring compared to advanced monitoring.37 Moreover, it was shown that the presence of PVAs do not necessarily affect outcomes in patients with CHRF.39 Conversely, Adler et al. showed that actively titrating NIV to minimize PVA and sleep-disordered breathing decreased morning dyspnea and increased patient-comfort.40
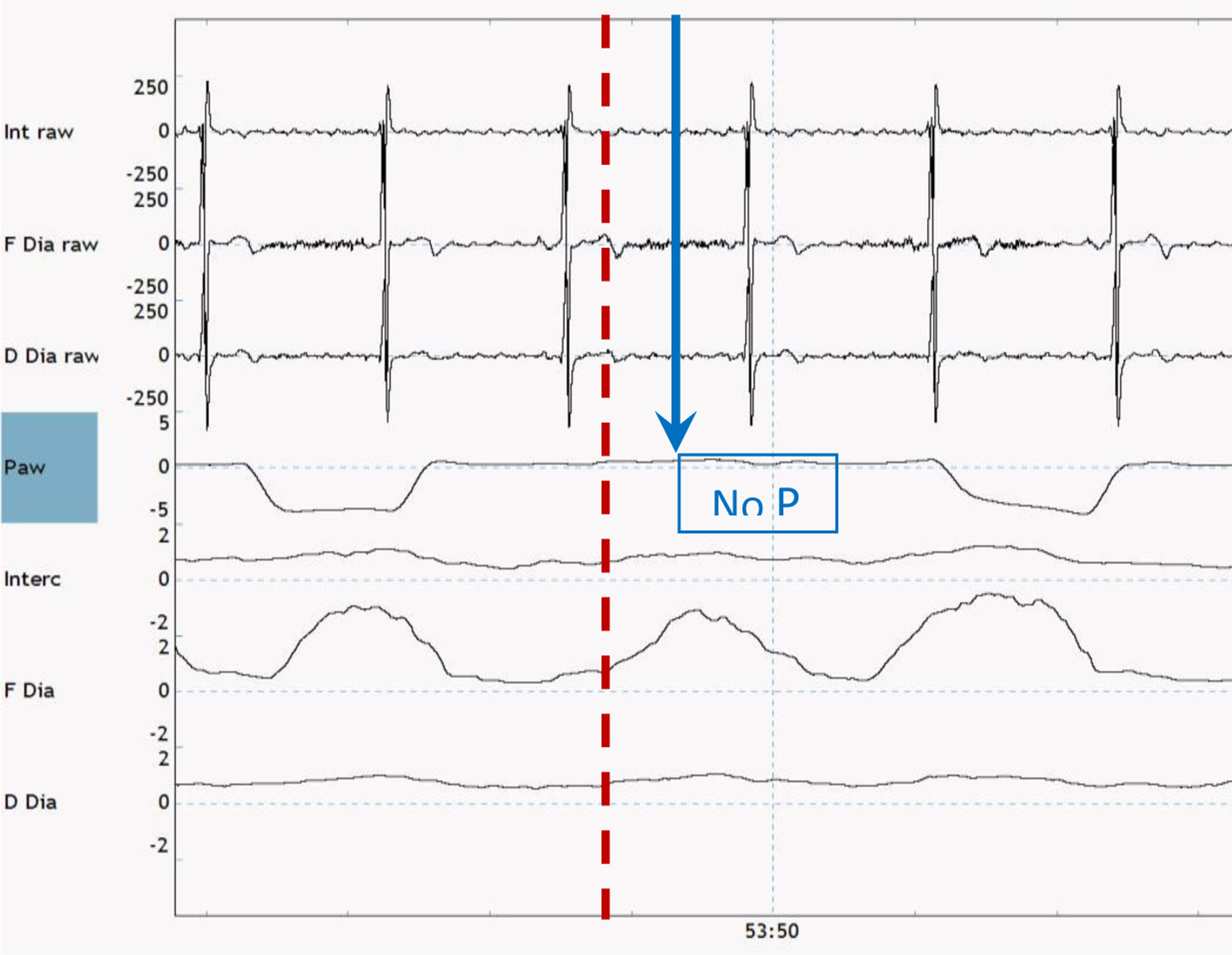
There are multiple methods available to monitor PVA noninvasively. Theoretically these methods can also be used at home, but in clinical practice, are still quite difficult to perform and have a high chance of technical errors in the unsupervised situation. A method propagated by the SomnoNIV group is the use of visual analysis of polygraphic (PG) tracings of chest and abdominal movement to detect patient effort and compare this with pressure and flow tracings from an external pneumotachograph.41 However, this method is quite complex, time-consuming and expensive, and in many centers only feasible when a sleep analysis is needed anyway. Furthermore, the tracings of chest and abdominal movement are not the gold standard to detect patient effort. In this respect, a more precise method to detect PVA might be to compare pressure waveforms with the patients’ own respiratory activity measured with electromyography (EMG).39,42 An example of surface EMG combined with airways pressure to detect an ineffective effort is shown in Fig. 2. For both methods, the processing of the signals and detection of methods still requires a lot of time and effort; the development of reliable automatic methods to detect relevant events would be very welcome.
The available knowledge about the effect of long-term NIV on lung mechanics and physiology and respiratory muscle functioning and mechanics is limited. Some smaller trials from Belgium showed that NIV in COPD can improve ventilation-perfusion matching.43 This knowledge gap is a pity as more insight in working mechanics might lead to better patient selection and a better funded approach towards the optimal settings. However, for long-term home NIV we need non-invasive monitoring methods suitable for the awake, moving patient in the home situation.
In conclusion, the more advanced monitoring described above is still an area of research. Methods need to be developed further to enable reliable home monitoring and automatic processing of signals and research has to show its eventual clinical value.
Follow-up of HMVFollow-up of HMV patients is a black box; there are no evidence based guidelines describing how and how often ventilatory support should be monitored. Moreover, follow-up frequency might differ between patient groups; patients in rapidly changing conditions (children) or patients with a rapidly progressive disease (ALS/COPD) might need more frequent follow-up compared to the slower or non-progressive diseases. Furthermore, there is no consensus about which minimal set of parameters should be monitored. Finally, also in the follow-up of patients, telemedicine might bring attractive alternatives.
The number of studies on the use of follow-up telemonitoring in patients on HMV is limited. Furthermore, telemonitoring/tele-medicine is a very broad concept; the exact way (what is monitored, how often, which equipment is used, which actions are taken upon monitored data) largely influences the eventual results and benefits. Vitacca et al. enrolled 240 patients with chronic respiratory failure due to different underlying diseases in a study investigating tele-assistance composed of remote oxygen saturation monitoring and scheduled and unscheduled tele-consultations.44 Sixty-two percent of the patients used home ventilation (43% NIV; 20% invasive mechanical ventilation). They showed that the number of hospitalizations per month was significantly fewer in the tele-assistance group and in COPD there were fewer acute exacerbations as compared to the standard care group. The tele-assistance team received mean 4.2 pulse oximeter reports per months and the number of requested calls (0.5/month) on top of the scheduled calls (2.42/ month) per patient per month was relatively low. Of note, of the 351 patient screened for the study, 111 patients (56% COPD) were excluded because of reduced cognitive status, insufficient family cultural requisites and lack of home prerequisite for tele-assistance. Chatwin et al. randomised 39 patients with severe COPD on LTOT or NIV (84%) to a rather extensive telemonitoring of physiological parameters and symptoms or to standard care, but failed to show benefit in terms of time to hospital readmission or HRQoL during a 6-month time period.45 In this study the number of home visits increased as well as the admission rate for acute exacerbations. Of note, the patients in this study seemed to be much more worried by their tele-monitoring, as the number of telephone consultations and alerts due to SpO2 was high (29 consultations per months and 187 alerts per month). This controversy between studies highlights the fact that the content of the tele-monitoring intervention (what is measured; how frequently) greatly influences the results; while sufficient monitoring might improve outcomes probably over- extensive monitoring without a self-management plan might only reduce patient self-efficacy.
While the above discussed studies monitored among others oxygen saturation and symptoms, in patients on HMV it is also possible to remotely monitor ventilator data, like compliance, tidal volume, breathing frequency etc. Studies have been performed focusing on the potential of predicting exacerbations prematurely by machine readouts through tele-monitoring.26,27 This seems an intriguing objective, as these studies did show a reduction in hospital readmissions and severe exacerbations.
Crucial to the success of the use of telemonitoring in follow-up of patient on HMV is the development of a good system and good algorithm to pick up the right physiological changes/parameters that really predict worse outcomes. Furthermore, this algorithm should lead to correct actions of the patients and/or caregivers. Further studies are needed to show the benefit with regard to patient-related outcomes and costs. In the future, telemonitoring follow-up might lead to personalized treatment, selecting patients that need more or other care earlier than planned or on the other hand, avoid unnecessary care in patient who do fine on their own.
Conflicts of interestMarieke Duiverman has no conflicts of interest for this manuscript.
None. Marieke Duiverman completed this review as employee of the University Medical Center Groningen. No additional funding was provided.