As neuroinflammation is increasingly recognized to be associated with neurodegenerative diseases, accumulating evidence suggests viral or bacterial infections are potential causes of Parkinson's disease (PD). Tuberculosis (TB) infection is associated with various diseases linked by chronic infections and inflammation. However, there are few studies which evaluated an association between TB and incident PD. In this context, we estimated PD incidence among TB survivors compared to that of the general population.
We performed a population-based retrospective cohort study using the Korean Nationwide Health Insurance System (NHIS) database. We included 67,475 TB survivors who underwent health screening within two years before TB diagnosis between 2010 and 2017, and 67,475 age- and sex-matched controls. The cohort were followed up for incident PD from one year after TB diagnosis to the date of PD event, date of death, or until the last follow-up date (December 31, 2018), whichever came first.
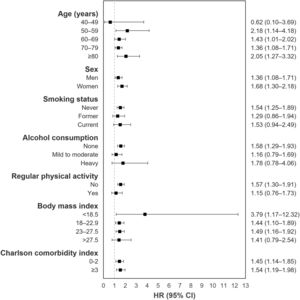
The mean follow-up duration was 3.6 and 3.7 years for TB survivors and matched controls, respectively. In total, 0.5% of TB survivors (363/67,475) and 0.3% of matched-control subjects (228/67,475) developed PD, with incidence rates of 1.5 and 0.9 per 1,000 person-years, respectively (Table 1). TB survivors had a higher risk of PD (adjusted hazard ratio 1.45, 95% confidence interval=1.22–1.73) compared with matched control. Fig. 1 shows the results from stratified analyses regarding the risk of PD among TB survivors with the matched control group as the reference after adjusting for potential confounding variables in all subgroups. TB survivors continued to have higher PD incidence in all subgroups compared with matched controls (P for interaction >0.05 for all).
Hazard ratios and 95% confidence intervals for the incidence of Parkinson's disease in tuberculosis survivors compared to the matched control group.
IR, incidence rate per 1,000 person-years; HR, hazard ratio; CI, confidence interval; TB, tuberculosis.
Model 1: crude model.
Model 2: adjusted for age, sex, socioeconomic position (income level and place of residence), smoking, alcohol consumption, regular physical activity, and body mass index.
Model 3: Model 2 + adjusted for Charlson comorbidity index.
Model 4: Model 3 + adjusted for competing risk for mortality.
Subgroup analysis for Parkinson's disease incidence in tuberculosis survivors compared with the matched control group, HR, hazard ratio; CI, confidence interval, Adjusted for age, sex, socioeconomic position (income level and place of residence), smoking, alcohol consumption, regular physical activity, body mass index, Charlson comorbidity index, and competing risk for mortality.
Although the role of TB infection in PD is far from being confirmed, both diseases share some inflammation mechanisms via interleukin-6, tumor necrosis factor, interleukin-1β, and matrix metalloproteinase.1 When an infectious bacterial agent is inhaled, swallowed, or enters the eyes, it induces abnormally enhanced cytokine production, so-called cytokine storm.2 Circulating dysregulated cytokines travel through the circulatory system to the brain, inducing fenestration in the blood brain barrier.2 In the brain, cytokines induce activation of resident microglia and astrocytes that induce neuronal damage.2 Dopaminergic neurons aggregate α-synuclein into Lewy Bodies and oxidative-stress-induced cellular damage.2 Conversely, in a mouse model, vaccination with neuronal antigens in complete Freund's adjuvant, which contains heat-inactivated M. tuberculosis or the TB vaccine strain Bacilli Calmette-Guerin partially protected against PD-associated neuronal death.3 Together, these data suggest that immune response to TB may trigger or exacerbate neuroinflammation.
Additionally, several genes confer susceptibility to both TB infection and PD.4 Leucine Rich Repeat Kinase 2 (LRRK2) mutation, the most common genetic cause of PD, might result in dysregulation of peripheral immune responses to pathogens and inflammation, which can have long-term consequences such as loss of dopaminergic neurons.5 Parkin RBR E3 ubiquitin protein ligase (PARK2) mutation is also a well-known genetic risk factor for PD. Polymorphisms in the regulatory region of PARK2 result in reduced expression of the parkin protein.6 The parkin protein is an ubiquitin ligase in mitophagy, which is a key innate defense mechanism against invading microbes.7 Therefore, mutations in LRRK2 or PARK2, which imply an impaired innate defense mechanism against TB infection may also lead to increased PD susceptibility.
We also noted that TB survivors were associated with increased PD risk even after various stratifications. These findings may highlight the causal relationship between TB and PD development. Interestingly, TB survivors who engaged in regular physical activity had no increased PD risk compared with healthy controls. Although the clinical significance of this finding is unclear due to the small difference, this study supports evidence that physical activity has a preventive impact on PD, even in high-risk groups like TB survivors
One major strength of our study was the reliability of the data obtained from the TB control system of Korea. In 2000, the Tuberculosis Notification Information System was established, and since 2001 all patients with TB have been required to register their cases electronically. Other strengths are that the NHIS database includes data from the entire Korean population, which results in almost complete follow-up. Our study also had some limitations. First, because this study is based on data that were not originally designed for studying the association between TB and PD, we were not able to control acute or chronic infections other than TB. Further studies are needed to verify the association between TB and PD. Second, because we used claims data, it is possible that PD was undetected, overestimated, or misdiagnosed. However, we also used a special registration code for PD, so that our definition of PD would have high accuracy.
In conclusion, we demonstrated that TB survivors exhibited a higher risk of PD compared to the general population, indicating that TB is an important infectious factor associated with increased incidence of PD.
FundingThis research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, Information and Communications Technologies (MSIT) (NRF-2020R1F1A1070468 and 2021M3E5D1A01015176).
This study was performed using the database from the National Health Insurance System, and the results do not represent the opinion of the National Health Insurance Corporation.