The management of unresectable stage III non-small cell lung cancer (NSCLC) is clinically challenging and there is no current consensus on optimal strategies. Herein, a panel of Portuguese experts aims to present practical recommendations for the global management of unresectable stage III NSCLC patients.
MethodsA group of Portuguese lung cancer experts debated aspects related to the diagnosis, staging and treatment of unresectable stage III NSCLC in light of current evidence. Recent breakthroughs in immunotherapy as part of a standard therapeutic approach were also discussed. This review exposes the major conclusions obtained.
ResultsPractical recommendations for the management of unresectable stage III NSCLC were proposed, aiming to improve the pathways of diagnosis and treatment in the Portuguese healthcare system. Clinical heterogeneity of patients with stage III NSCLC hinders the development of single standardised algorithm where all fit.
ConclusionsA timely diagnosis and a proper staging contribute to the best management of each patient, optimizing treatment tolerance and effectiveness. The expert panel considered chemoradiotherapy as the preferable approach when surgery is not possible. Management of adverse events and immunotherapy as a consolidation therapy are also essential steps for a successful strategy.
Locally advanced stage III non-small-cell lung cancer (NSCLC) includes tumours exhibiting extension into extrapulmonary structures, involving hilar or mediastinal lymph nodes, but with no evidence of distant metastasis.1 According to the 8th edition of the TNM Classification of Malignant Tumours (T – Primary Tumour, N – Regional Lymph Nodes, M- Distant Metastasis), stage III includes three subgroups: i) stage IIIA comprises T4 N0 M0 and T3/4 N1 M0 tumours as well as T1/T2 N2 M0 tumours; ii) stage IIIB tumours are either T3/T4 N2 M0 or T1/T2 N3 M0 and iii) stage IIIC involves T3/T4 N3 M0 tumours.2 The new classification unveils the anatomical extent and the heterogeneity of the disease when diagnosed in this stage, that accounts for approximately 20–25% of NSCLC.3 Significant advances emerged in imaging, diagnostic and staging techniques including new generation computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS) and video-assisted thoracoscopic surgery (VATS), which are now routinely used in lung cancer management.4
The development of combined treatments for unresectable stage III NSCLC has led to a significant improvement of 5–10% in the 5-year overall survival (OS) rate as opposed to with radiotherapy alone. Concurrent chemoradiotherapy (CCRT) provided a 5-year OS of 16% when compared to 9% with sequential chemoradiotherapy (chemotherapy followed by radiotherapy - SCRT).5 A complex multimodal treatment strategy, including surgery, radiotherapy and systemic therapy should be discussed by an experienced multidisciplinary team (MDT).
Recent research in oncology has led to an expanded reach and impact of immune checkpoint inhibitors as part of a frontline treatment strategy in metastatic NSCLC. Regarding the treatment of locally advanced disease, until the PACIFIC trial, no improvements were attained, with the standard treatment being definitiveCCRT.3
A panel comprising Portuguese lung cancer experts discussed aspects related to staging and treatment of unresectable stage III NSCLC considering current evidence. This document aims to present the outcome of the discussion, proposing practical recommendations for the global management of unresectable stage III NSCLC patients.
MethodologyA panel of 17 Portuguese physicians with recognised clinical expertise (8 pulmonologists, 3 radiation oncologists, 2 thoracic surgeons, 2 oncologists, 1 pathologist and 1 radiologist) reviewed the strengths and limitations of available evidence as well as the constraints of clinical daily practice, in order to elaborate practical recommendations for the clinical management of unresectable stage III NSCLC in Portugal. To overcome the limitations of a round table discussion in which members of the expert committee with stronger opinions may dominate over less assertive ones, an informal qualitative method was implemented in order to determine the percentage of consensus. Experts were asked to answer anonymously to a survey, indicating the level of agreement regarding the statements presented. In addition, a PubMed search was conducted to complement the panel discussion with the following terms: “lung cancer”, “diagnosis”, “staging”, “NSCLC stage III”, “locally advanced NSCLC”, “resectable and unresectable locally advanced NSCLC”, “neoadjuvant treatment” “chemotherapy”, “radiotherapy”, “concurrent chemoradiotherapy”, “sequential chemoradiotherapy”, “durvalumab”, “immunotherapy in stage III”. This manuscript included published articles in the last ten years prior to April 2020. Only articles published in English were reviewed. Up-to-date and evolving management guidelines related to NSCLC were selected as well as original research and reviews on the basis of their clinical relevance to each section of this manuscript.
DiscussionDiagnosis and staging of unresectable stage III NSCLCInitial evaluationThe majority of stage III patients present symptoms at their initial presentation, that can be tumour related (persistent coughing, chest/shoulder pain, breathing changes and haemoptysis) and/or systemic (loss of weight or appetite, fatigue).6,7 On this basis, the intention of the initial evaluation is to obtain enough information to reach a definitive diagnosis and establish a treatment plan with the fastest, safest and cost-effective strategy, taking into consideration patient's values and preferences. This evaluation comprises contrast-enhanced CT scan, complete laboratory evaluation and cardiorespiratory assessment,8,9 where patient's comorbidities and functional status must be also assessed.10
Adequate sampling for tumour diagnosisTechniques for sample collection should provide enough material for routine preparations (haematoxylin-eosin staining), immunohistochemistry (IHC) and molecular characterisation. Percutaneous transthoracic needle biopsy (TTNB), endobronchial biopsies and EBUS-transbronchial needle aspiration (EBUS-TBNA) provide adequate material if enough sample is collected.11 Cytology samples obtained from pleural effusions are sometimes highly cellular and can also be used for diagnosis, programmed cell death ligand-1 (PD-L1) expression determination12 and molecular characterisation.13 Samples should be of the highest quality and tumour cell purity as possible to permit adequate deoxyribonucleic acid (DNA) extraction.
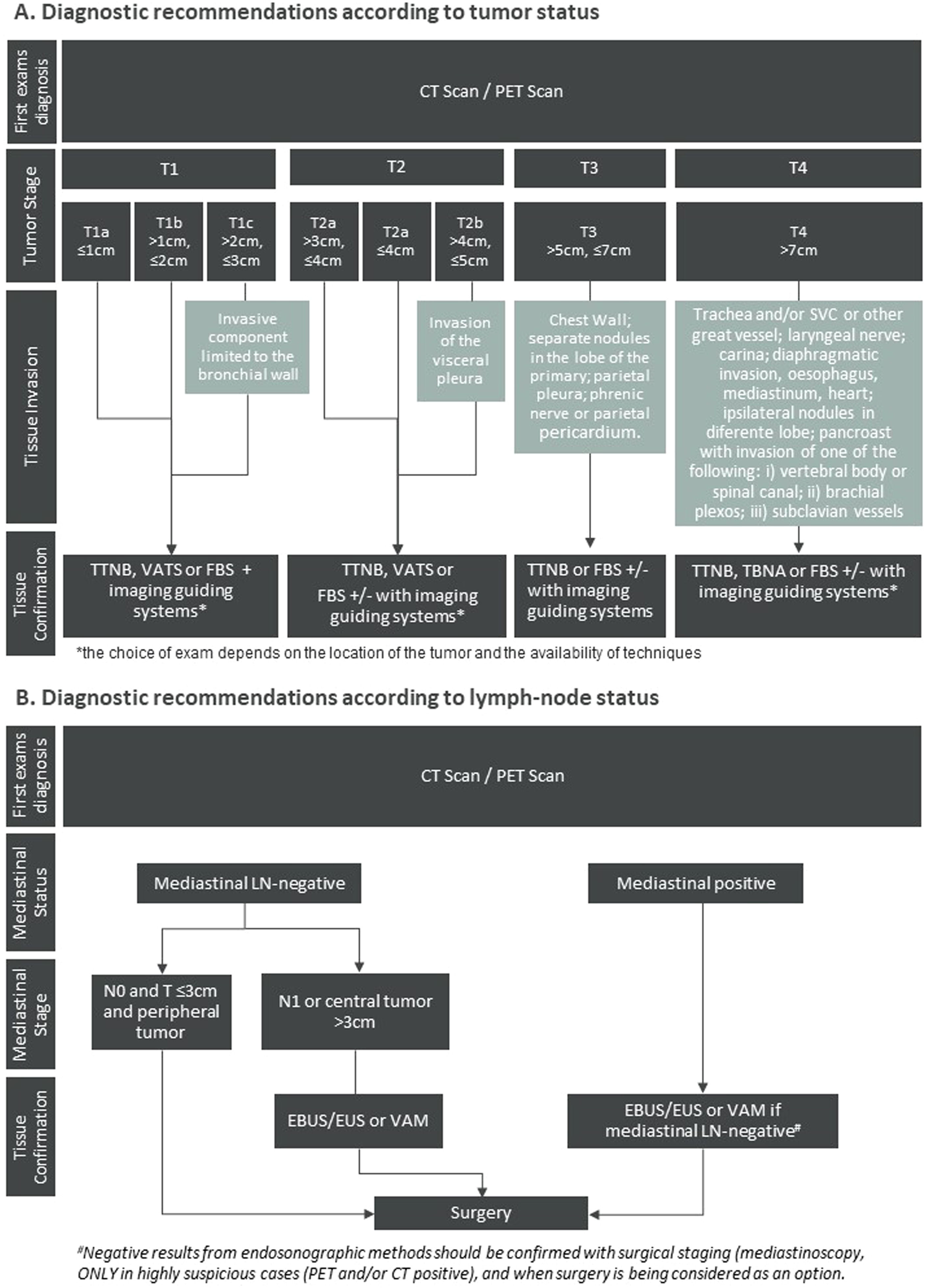
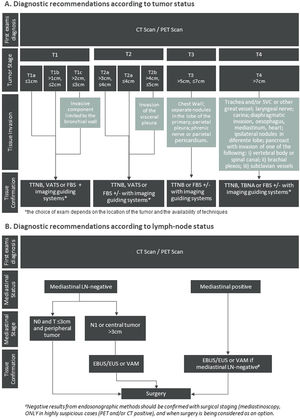
Diagnosis and staging strategyThe diagnostic recommendations rely firstly on an imaging evaluation to identify the tumour location (Fig. 1A). Bronchoscopy is highly sensitive for central tumours and for peripheral lesions with the complementary use of imaging guiding systems, as radial EBUS, fluoroscopy, electromagnetic navigation (EMN) or computerised navigation systems.14 For peripheral lesions, accessed through the chest wall transthoracic biopsy guided by CT scan is considered a sensitive and easily available approach. Also, transthoracic puncture guided by ultrasound may be used for pleural-based lung lesions to obtain an adequate amount of tissue, increasing the diagnosis accuracy (Fig. 1A).15
Algorithm representing the practical recommendations for diagnosis of locoregional NSCLC according to (A) tumour and (B) lymph-node status. The recommendations present in diagram B were adapted from Leyn et al. Eur J Cardio-Thoracic Surg. 2014.16
Abbreviations: CT = computed Tomography; EBUS = endobronchial ultrasound; EUS = oesophageal ultrasound; FBS = flexible bronchoscopy; LN = lymph Node; MRI = magnetic resonance imaging; PET = positron emission tomography; TBNA = transbronchial needle aspiration; TTNB = transthoracic needle biopsy; VAM = video-assisted mediastinoscopy; VATS = video-assisted thoracoscopic surgery.
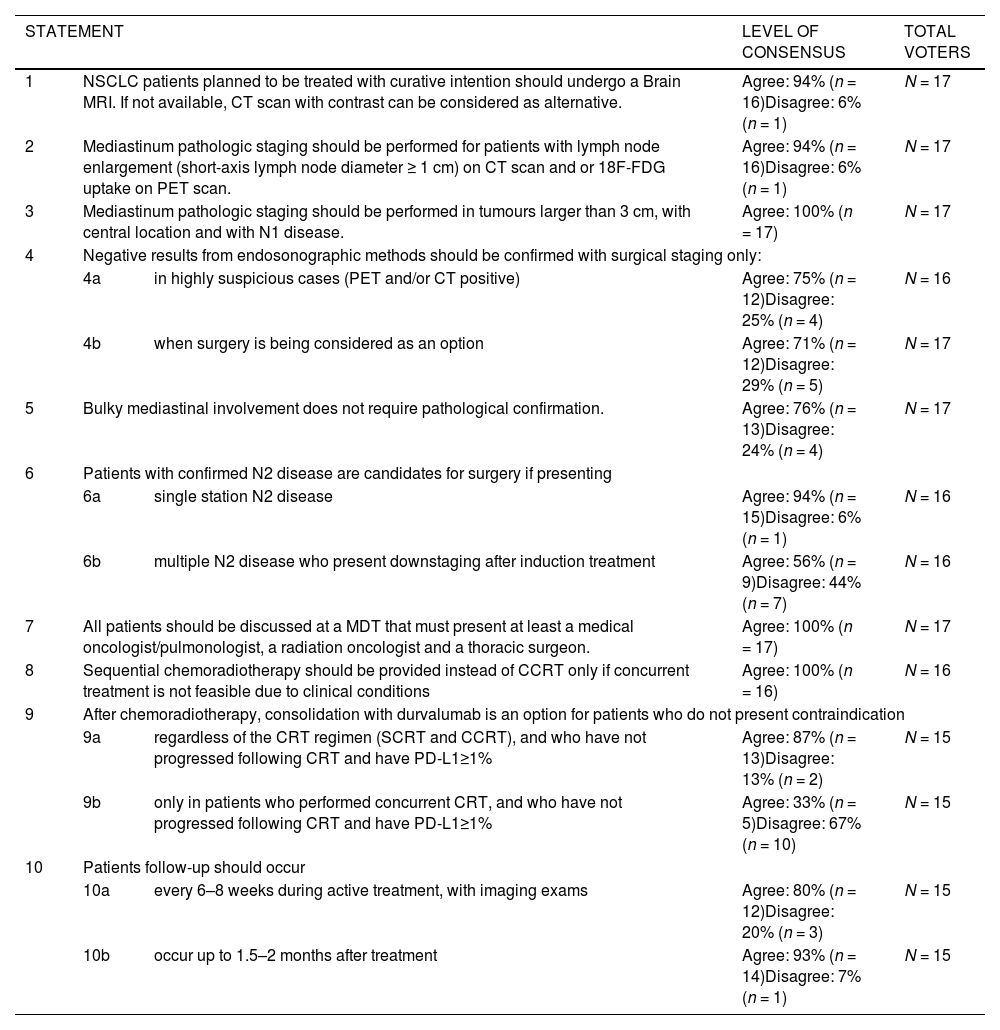
Accurate staging improves patient outcomes. The recommendations state that every patient suspected of having lung cancer should undergo a contrast-enhance thoracic CT scan and PET-CT.16 Thoracic MRI may be necessary and complementary for the assessment of lung cancer tumour invasion of the chest wall,17 brachial plexus, superior sulcus, diaphragm and great blood vessels (in cine mode).18 If PET scans are unavailable, bone scan and abdominal CT are reasonable alternatives. All NSCLC patients planned to be treated with curative intention should undergo a brain MRI or, if MRI is not available, a contrast-enhanced CT scan.10
Staging of the mediastinum is recommended for patients with lymph node enlargement (short axis diameter ≥ 1 cm) on CT scan and/or 18F-FDG uptake on PET scan, in the absence of distant metastasis. Also, tumours larger than 3 cm, with central location and with N1 disease should undergo mediastinal staging (Fig. 1B).
For the assessment of mediastinal node involvement, minimally invasive needle techniques (EBUS-TBNA, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or combined EBUS/EUS) are the first choice (Fig. 1B). These recommendations are in line with De Leyn et al.16 which reflects the most updated guidelines from European Society of Thoracic Surgeons. Negative results from endosonographic methods should be confirmed with surgical staging (mediastinoscopy, VATS) only in highly suspicious cases (PET and/or CT positive), and when surgery is being considered as an option, given that after a negative EBUS-TBNA the prevalence of mediastinal lymph nodes is low (near 5%).19 On the other hand, bulky mediastinal involvement does not need pathological confirmation.
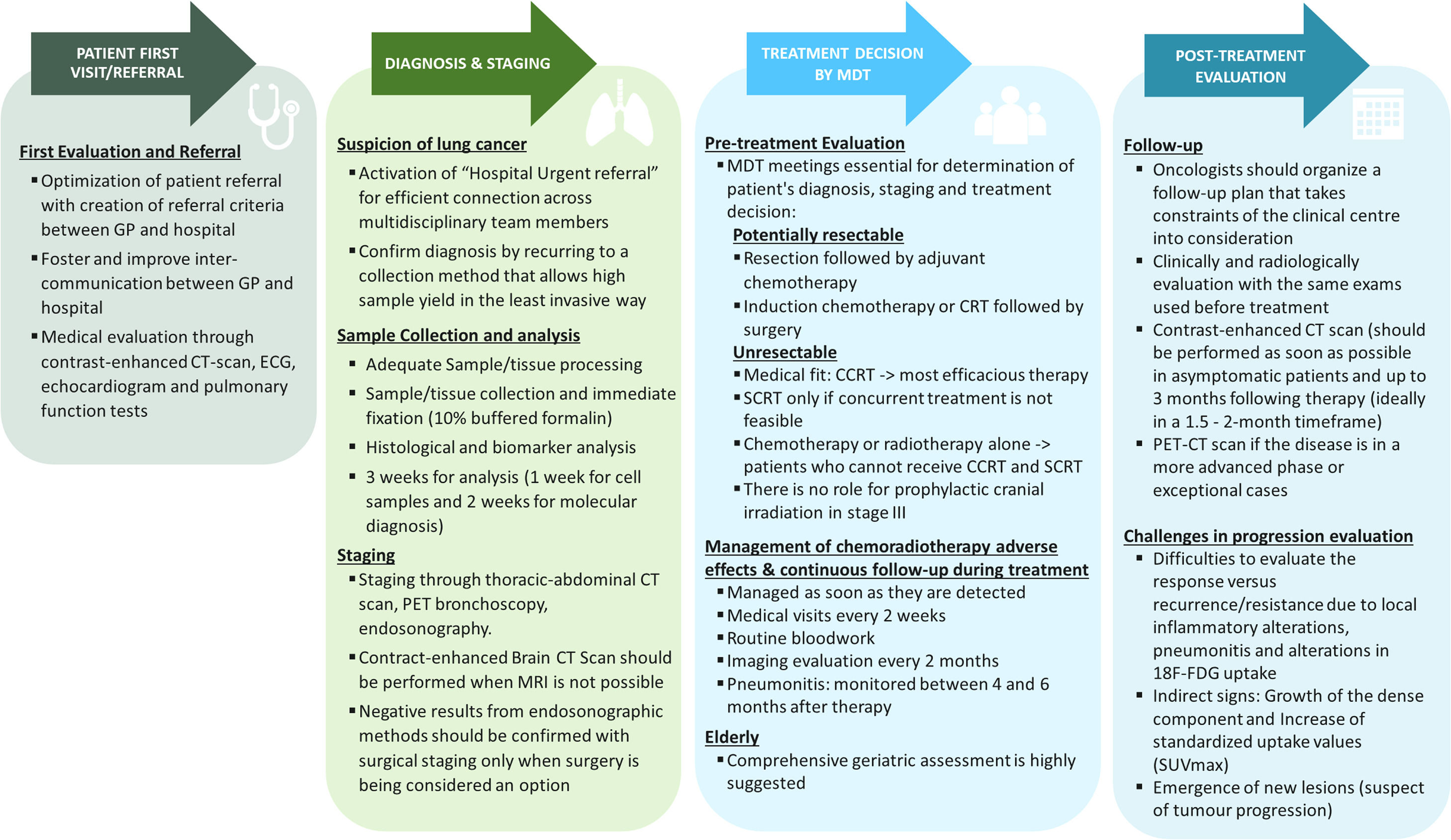
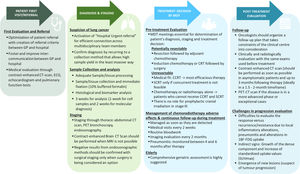
Practical recommendations from patients first visit to diagnosis, staging, treatment decision and follow-up are summarised in Fig. 2.
Highlights the overall management of unresectable Stage III NSCLC. Abbreviations: BRAF = B-Raf proto-oncogene serine/threonine kinase; CCRT = Concurrent chemoradiotherapy; CRT: chemoradiotherapy; CT = computed tomography; ECG = Electrocardiogram; EGFR = Epidermal growth factor receptor; GP = General practitioner; LA-NSCLC: Locally advanced non-small cell lung cancer; MRI = magnetic resonance imaging; NSCLC = Non-small cell lung cancer; PD-L1 = Programmed cell death ligand-1; PET = Positron emission tomography; ROS1 = c-ros oncogene 1 receptor tyrosine kinase; SCRT: Sequential chemoradiotherapy; SUVmax = Maximum standardized uptake values; TTF1 = Thyroid transcription factor 1.
In early-stage NSCLC, surgical resection is the treatment of choice.20 However, the determination of resectability should be discussed by the MDT21 which includes certified thoracic surgeons who perform lung cancer surgery as a prominent part of their practice. Surgery should always be considered when R0 surgical resection is feasible (resection margins with no microscopic evidence of tumour cells; systematic nodal dissection or lobe-specific systematic nodal dissection; nodule capsule removed separately and of lymph nodes located at the margin of the main lung must be without extracapsular tumour invasion; highest mediastinal lymph node must be tumour free22 as is usually reserved for N1 or N0 patients. However, patients with preoperative pathological proven N2 disease are candidates for surgical multimodality treatment under two circumstances: Single station N2 disease or patients with multiple N2 disease who present downstaging after induction treatment).16 Under this situation, induction treatment is considered to decrease the extent of tumour involvement, reducing the complexity of surgical resection and thus increasing resectability but also to improve local control of the disease and eliminate micrometastases.23 The group of experts also considered that after assessment of resectability, a precise clinical/functional evaluation is required prior to surgery, underlying the need to perform functional evaluation, namely: general health and performance status (PS) assessment, cardiac function, pulmonary mechanics, diffusion capacity, exercise capacity, extent of resection and age.20,24
Limits of resectabilityThe panel propose the following limits for resectability in stage III, that should not be exceeded:
- ○
Single station N2 disease up to 3 cm in diameter where other nodal stations have been biopsied and proved to be benign,21
- ○
Some multiple N2 disease after induction therapy, when there has been nodal downstaging and a pneumonectomy can be avoided,21
- ○
T3 with lymph nodes classified as N0 and N1 in addition to the involvement of the main bronchus (less than 2 cm from the carina, but without invasion of the carina),21
- ○
T3 with chest wall involvement (including superior sulcus tumour) could receive neoadjuvant chemoradiotherapy (CRT) followed by surgery,
- ○
T4N0 tumours where nodal disease had been excluded by invasive methods and when a R0 resection is considered to be feasible.21
The standard treatment for unresectable stage III NSCLC is CCRT. However, for unfit patients SCRT remains an option, and for those with a poor PS the option could be single treatment radiation. The treatment decision depends on several factors: i) patient age, ii) PS and comorbidities, iii) tumour location and total tumour volume. Chemoradiotherapy may be offered following proper individual assessment.25–27 Treatment should start as soon as possible according to the available resources and best clinical practices.28 After CRT, consolidation with immunotheraphy is an option for patients without contraindications, with recent evidence from the phase III PACIFIC trial showing that immunotherapy is a promising therapeutic strategy for consolidation therapy in stage III patients.29,30
Concurrent vs sequential chemoradiotherapyData from several phase III trials comparing SCRT to CCRT was abridged in a meta-analysis leading to the conclusion that CCRT is considered the preferred treatment for patients who are fit, as it leads to higher 5-year survival rates (with an absolute benefit of 4.5% at 5 years).1,31,32 In CCRT, depending of the selected protocol, at least two cycles of platin-based chemotherapy should be performed during radiotherapy. Ideally, radiation treatment should start on day 1 of chemotherapy or within the first 2 cycles.33 CCRT with a platinum-doublet chemotherapy improve progression free survival (PFS) in comparison with a single-agent chemotherapy.34 The SCRT regimens comprise four cycles of platinum-based chemotherapy that should be performed before radiation therapy, which should start up to 4 weeks after the end of chemotherapy.21 Most studies of CCRT and SCRT use cisplatin and etoposide or cisplatin and vinca alkaloid (typically: cisplatin / vinorelbine), carboplatin and paclitaxel (weekly) or cisplatin and pemetrexed if non-squamous histology.
Ideally, radiotherapy treatment should not exceed 7 weeks. A total dose of 60–66 Gy administered in 30–33 fractions once daily, 5 times a week, is the standard treatment for both regimens (CCRT and SCRT).35,36 Intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) techniques should be preferred, as they compare favourably with 3D treatment options. The advantages of these techniques lies in safe dose escalation, providing optimal doses for tumour irradiation while sparing the surrounding tissue.37 In addition, IMRT and VMAT have low toxicity and decrease the incidence of acute and late onset adverse effects and, therefore, decrease the need for treatment cessation.38,39
ImmunotherapyRecent advances in the understanding of tumour immunology led to the development of new therapeutic agents, such as immune checkpoint inhibitors.40 This strategy is already part of the standard treatment in more advanced stages.41
Rationale for immunotherapy following chemotherapy is based on the knowledge that chemotherapy is able to upregulate tumour antigens.42 Moreover, radiation on tumour cells leads to priming and activation of cytotoxic T cells, facilitating the recruitment and infiltration of immune cells in the residual tumour.43 European Medicines Agency (EMA) approved durvalumab in adult patients locally advanced, unresectable non-small cell lung cancer whose tumours express PD-L1 on ≥ 1% of tumour cells and whose disease has not progressed following platinum-based chemoradiation therapy.44
This approval was based on the results of the phase III PACIFIC trial, which included patients with unresectable stage III NSCLC whose disease did not progressed after two or more cycles of platinum-based CCRT, and received their last radiation dose within 1–42 days before randomisation.29 Durvalumab was compared to placebo for up to 12 months after CCRT. The results at a median follow-up of 33.3 months showed that the median OS had still not been reached in the durvalumab arm compared with 29.1 months in the placebo arm (stratified HR, 0.69; 95% CI 0.55 to 0.86). The immunotherapy should start as soon as possible (within 1 to 2 weeks) as benefits seem to be greater.
Concerning adverse events, pneumonitis was reported to be higher in patients who received durvalumab, however grade 3 or 4 pneumonitis was similar in both groups: 1.9% in the durvalumab arm and 1.7% in the control group. Moreover, pneumonitis resulting from radiation contributed to durvalumab discontinuation in 1.3% of patients, as in the placebo arm.30 Hui et al.45 reported that toxicity was manageable and quality of life was not compromised by adding 12 months of durvalumab after standard CCRT.
Although durvalumab is approved for use in Europe, the label is limited to patients whose tumours express PD-L1 on ≥ 1% of tumour cells and this restriction was based on a post hoc analysis consisting of a small sample size that prevent robust conclusions regarding OS in patients with PD-L1 expression <1%. This analysis has been focus of attention of a panel of international lung cancer experts46 but will not be further discussed in this document given that local reimbursement of durvalumab also excluded patients with PD-L1 expression <1% .47,48
The Portuguese experts agreed that durvalumab should be recommended as consolidation treatment after CRT for unresectable stage III. The majority of experts (87%) approves this recommendation for patients who do not present contraindications, regardless of the regimen (SCRT or CCRT) and who have not progressed following CRT and have PD-L1≥1% (Table 1).
Consensus on management of unresectable stage III NSCLC.
Abbreviations: 18F-FDG = 2-deoxy-2-(18F)fluoro-D-glucose; CCRT = concurrent chemoradiotherapy; CT = computed tomography; MDT = multidisciplinary team; MRI = magnetic resonance imaging; NSCLC = non-small cell lung cancer; PD-L1 = programmed cell death ligand-1; PET = positron emission tomography; SCRT = sequential chemoradiotherapy.
During the transition from active treatment to post-treatment follow-up, a defined strategy with follow-up schedules should be implemented, aiming at maximization of the benefit. Thus, while during treatment tumour re-evaluation should be performed every 6–8 weeks (timed to coincide with the end of a cycle),49 post-CRT evaluation is usually performed within 1.5–2 months, when the maximal therapeutic effect is expected.50 CRT response should be routinely evaluated by plain X-ray51 or preferably by contrast-enhanced CT scan.52 These results should be compared to those obtained before treatment. Also, a PET-CT scan to evaluate tumour metabolic activity is recommended when abnormalities, particularly if suggesting progression of the disease, are detected on CT scans.35 However, changes in 18F-FDG uptake that normally occur between 8 and 12 weeks after radiation therapy should be taken into account, as they are potential confounding factors in this setting.53 Moreover, many benign conditions (such as atelectasis, consolidation, and radiation fibrosis) are difficult to differentiate from neoplasm because areas previously treated with radiation therapy can remain 18F-FDG avid for up to 2 years.
Adverse events (e.g., pneumonitis) should be immediately managed. Clinically, induced pneumonitis may hinder evaluation of response.54 The panel indicated that in the presence of indirect progression signs namely growth of the dense component, increase of maximum standardized uptake values (SUVmax) or the emergence of new lesions, histological confirmation of progression must be assessed.
Furthermore, a summary of the management of unresectable Stage III NSCLC is presented in Table 2 and agreement level between experts is presented in Table 1.
Recommendations for improvement of patient care – from early diagnosis to access to innovations.
Abbreviations: MDT = Multidisciplinary team.
Regardless of the disease stage, all patients with lung cancer should have access to treatment and supportive care options and be fully informed of their treatment options. A care plan must be developed for every patient to facilitate timely and effective information exchange. Services should collect data and measure patient experience to improve lung cancer care.
Considering the rapid evolution of lung carcinogenesis, the diagnosis of this disease shall be straightforward. The panel recommends that increased and high-quality information should be available to the public, targeted accordingly to age and literacy of the audience, focusing not only on the suspicious signs and symptoms but also on the link between this disease and tobacco use, as well as measures to encourage tobacco cessation (Table 2).
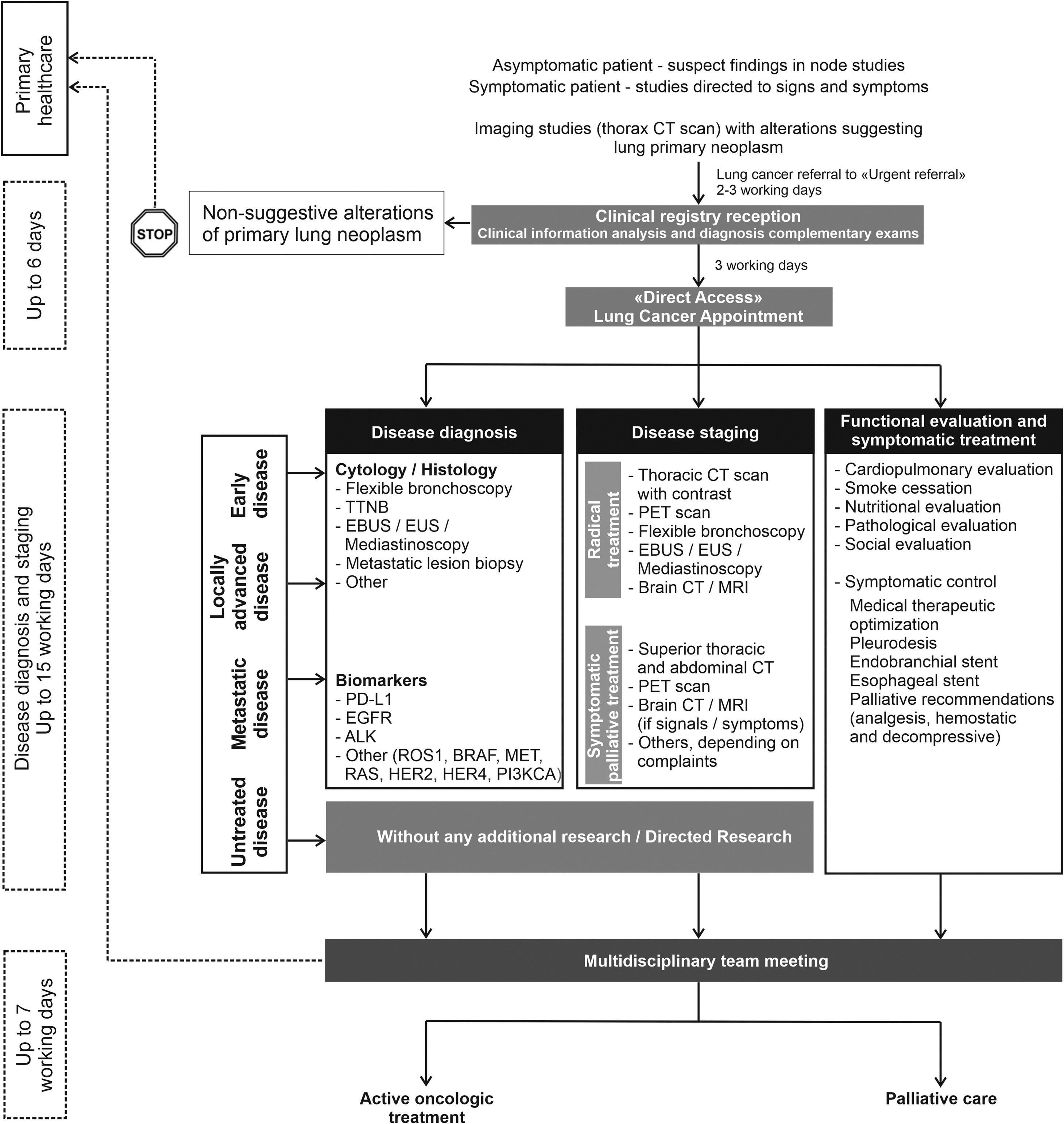
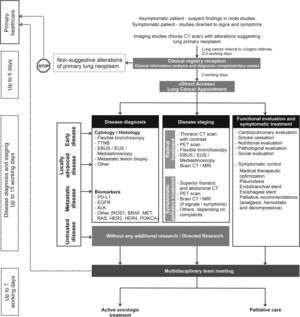
Multiple clinical practice guidelines recommend rapid evaluation of patients with suspected lung cancer.8,55 The panel recommendations are: 1) patients with an abnormal chest radiograph or a high suspicion of lung cancer based on clinical judgement must undergo chest CT scan within 2 weeks; 2) patients referred to a specialist or a diagnostic assessment program should expect a consultation within 2 weeks and, having confirmed the diagnosis, pathology results should be available within 2 weeks of the relevant procedure; 3) molecular evaluation and PD-L1 expression should be available within 2 weeks of specimen arrival at the laboratory and on time for treatment decision.56 To accomplish these goals, intra-institutional communication and organisation seems to be one of the most determining factors. The panel recommends the implementation of a preferential pathway for lung cancer patients that could facilitate and accelerate the diagnosis and staging, overcoming the constraints of the hospitals. This preferential pathway should embrace an ideal time from general practitioner (GP) referral to conducting complementary exams and initiation of treatment (fast-track for lung cancer) (Fig. 3).
Flowchart representing the fast-track referral pathway for NSCLC. Abbreviations: ALK = anaplastic lymphoma kinase; BRAF = b-raf proto-oncogene serine/threonine kinase; CT = computed Tomography; EBUS = endobronchial ultrasound; EGFR = epidermal growth factor receptor; EUS = oesophageal ultrasound; HER2 = human epidermal growth factor receptor 2; HER4 = human epidermal growth factor receptor 4; KRAS = kirsten rat sarcoma viral oncogene homologue; MRI = magnetic resonance imaging; PD-L1 = programmed death ligand 1; PET = positron emission tomography; PI3KCA = phosphatidylinositol 3-kinase catalytic subunit; ROS1 = c-ros oncogene 1 receptor tyrosine kinase; TTNB = transthoracic needle biopsy.
The panel also considers the implementation of an MDT essential to ensuring the best management approach for each patient and that all lung cancer patients should have their treatment discussed by a MDT. The MDT should comprise at least the three main specialists, a medical oncologist/pulmonologist, a radiation oncologist and a thoracic surgeon, with input from other experts, such as a pathologist and a radiologist, whenever possible. The MDT reports vary considerably but should ideally include the following: 1) demographics; 2) results of investigations, such as diagnosis and staging; 3) stage-dependant guideline recommended treatment; 4) treatment plan and reasons for nonadherence to guideline recommendations; 5) whether the MDT came to a unanimous or a majority decision; 6) common treatment adverse events and recommended management; and 7) a clear indication of the expected prognosis and follow-up strategy. The panel considers that access to innovation should be increased and harmonized across Portugal and similar to analogous European countries.
Patient-centred care (PCC) should be adapted to individual patient preferences, and values.57 PCC has a number of outlined dimensions: access to medical care, emotional support, involvement of relatives and friends, information and education.58 PCC can improve quality of care and patient satisfaction, as well as reduce healthcare costs (Table 2).59
ConclusionsPatients with stage III NSCLC are clinically heterogeneous and it is often difficult to fit them into a standardised algorithm. The expert panel believes that the route from diagnosis to therapy in unresectable stage III NSCLC involves several steps: i) timely diagnosis with priority access to diagnostic techniques and multicentre cooperation; ii) pre-treatment evaluation and staging by a MDT; iii) if surgery is not possible, the preferred treatment approach is a CCRT regimen, with SCRT and chemotherapy or radiotherapy alone being considered for unfit patients; iv) post-treatment evaluation for early identification of progression and management of adverse effects (e.g., pneumonitis); v) immunotherapy as a consolidation therapy should be considered if there is no progression and no signs of treatment-induced toxicity.
The continuation or maintenance of treatment in patients with unresectable stage III cancer is always urgent. Moreover, coordination with other specialised units in patient healthcare, such as nutrition, psychology, rehabilitation or geriatrics, can be of great importance to patients. Guidance from other units in terms of nutrition, psychology, rehabilitation or geriatrics would be beneficial to patients. This would facilitate treatment tolerance and also improve its outcomes.
The authors would like to acknowledge [Inês Coelho, Pharmaissues] for medical writing support that was funded by AstraZeneca in accordance with Good Publications Practice (GPP3) guidelines.