Whipple’s disease is a rare disorder caused by the Gram-positive bacterium Tropheryma whipplei (TW), formerly known as Tropheryma whippelii, that localizes initially in the lamina propria of small bowel with prominent gastrointestinal symptoms and weight loss.1 However, it can also cause a wide range of clinical manifestations, due to its spreading to other sites (i.e. endocardium, lymph nodes, central neurologic system, serum membranes)2 including pulmonary manifestations.3,4
We draw here to attention a quite unique case of Whipple disease complicated by lung involvement probably due to an immune reconstitution syndrome.
This refers to a caucasian 77-year-old man diagnosed with Whipple’s disease in 2015 (relapsed in 2018) treated with doxycycline and hydroxychloroquine then withheld because of gastrointestinal intolerance. In the past medical history, he was diagnosed with a type 1 myelomonocytic chronic leukemia, peripheral axonal neuropathy, dermatitis due to a possible immune reconstitution syndrome, osteoporosis due to steroid therapy treated with vertebroplasty, hypertension, aortic insufficiency, bilateral glaucoma, benign prostatic hypertrophy and intestinal-type gastric adenocarcinoma treated with endoscopic resection in 2019.
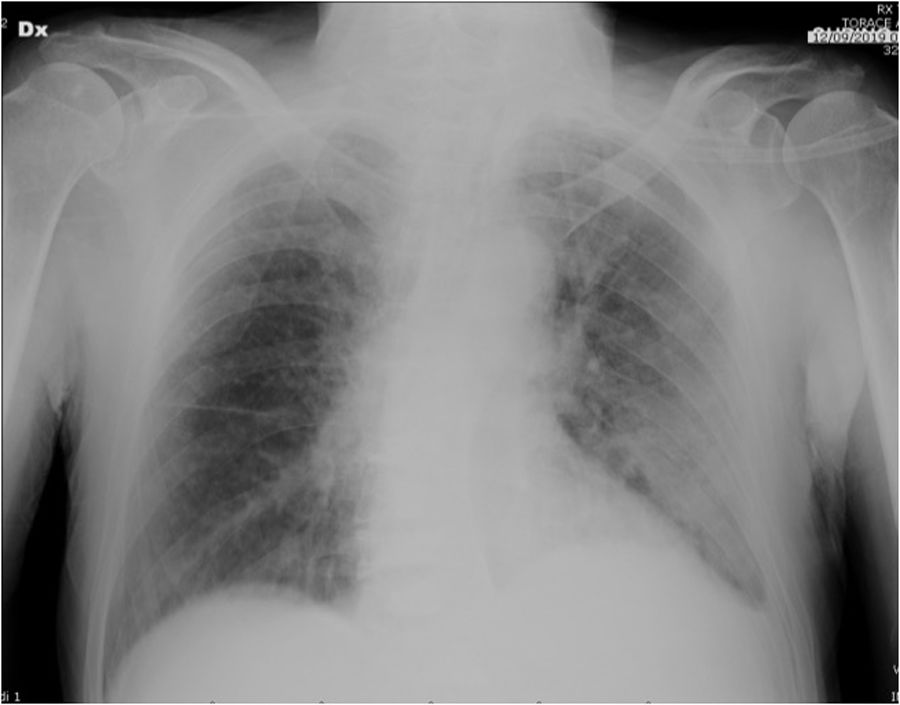
He was admitted on September 2019 to the emergency room due to high fever the night before, thoracalgia and arterial blood gases abnormalities (pO2 58mmHg, pCO2 29mmHg, pH 7.45); chest X-ray documented lung involvement with pleural effusion (Fig. 1). Vital parameters indicated mild hypotension (90/50mmHg), tachycardia (115bpm), tachypnoea (30/min), no alteration in body temperature. Blood tests showed anemia (6.8g/dl) mild elevation of C-Reactive Protein (2.8mg/dl), increased white cells (40.130mmc), Pro-calcitonin (34pg/ml), and creatinine (1.6mg/dl), while d-dimer was inconsistent and cardiac enzymes were negative. The patient was transferred to our ward on oxygen (2L/min flow by nasal prongs), drug regimen with piperacillin/tazobactam and prophylactic low molecular weight heparin was started, blood transfusion was also performed. Due to clinical signs of malabsorption, parenteral nutrition was instituted.
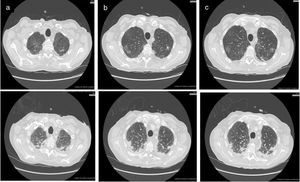
Chest ultrasound confirmed a bilateral pleural effusion, larger on the left, with extended B-lines. Bilateral apical opacities appeared at the chest-CT (Fig. 2 upper panels). The whole body exams performed to search for the most common pathogens (bacteria and respiratory viruses) were negative, and the bronchiolo-alveolar lavage also showed no significant isolates.
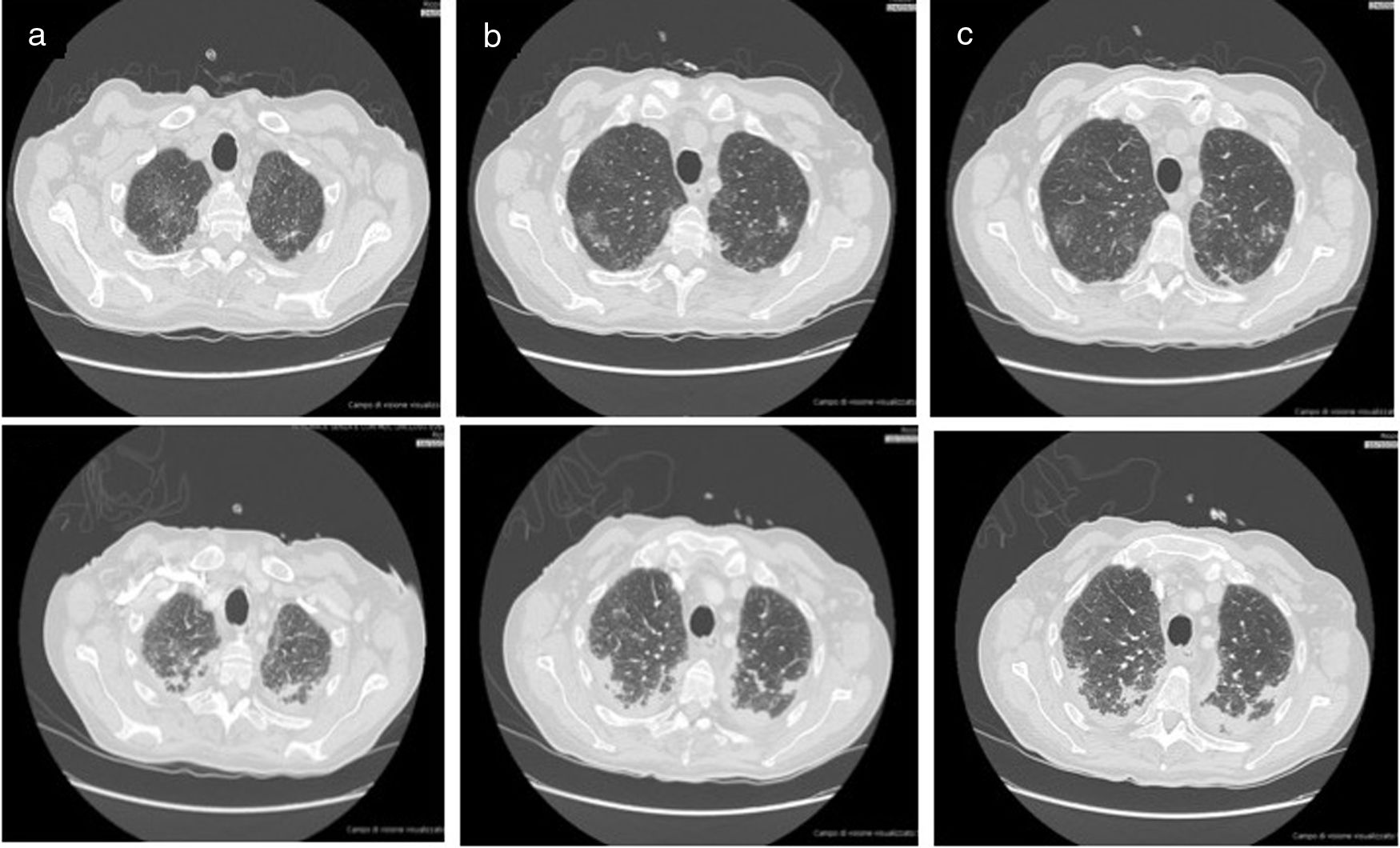
The first chest-CT performed in the ward demonstrated bilateral lung opacities with the appearance of ground-glass in the upper lobes (see upper panel 1 in different slices a to c).The second chest-CT performed after patient’s worsening demonstrated progression of the lung parenchimal opacities, with consolidations and reticolo-nodular interstitial thickening (see lower panel 2 in different slices a to c).
Following an initial clinical improvement, the patient condition deteriorated with fever, tachypnea and worsening of arterial hypoxemia (pO2 40mmHg with 2L/min oxygen) and impending fatigue of the respiratory system. Ceftazidime was added, taking the history of TW disease into account. A second chest-CT documented the worsening of the lung involvement (Fig. 2 lower panels) so patient was transferred to our respiratory intensive care unit, oxygen supplementation was set up to FiO2 50% and antibiotic regimen with cephalosporin was started.
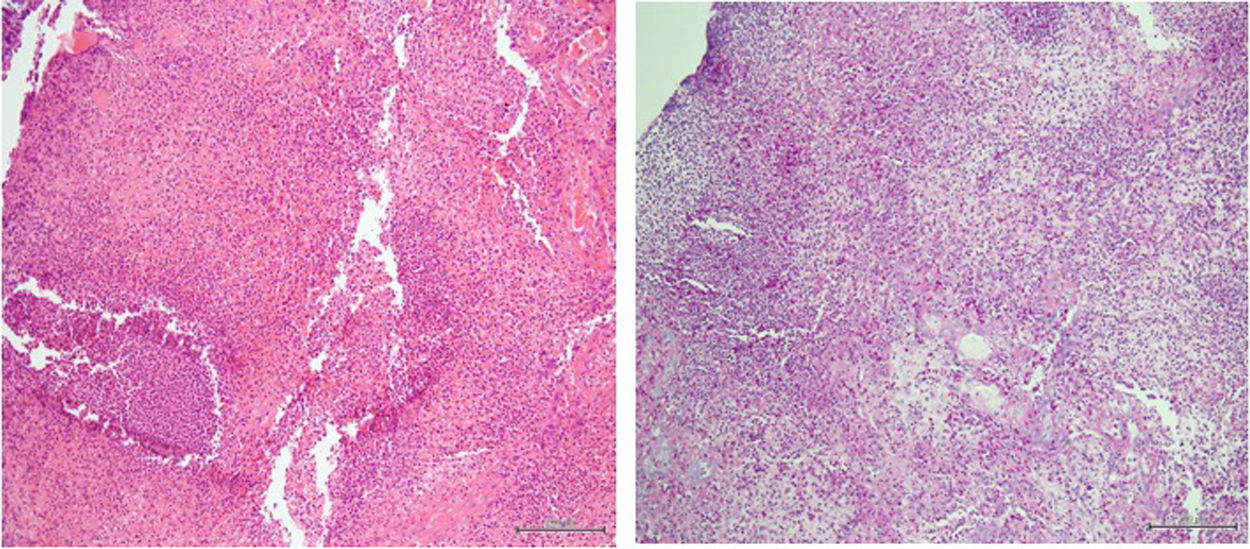
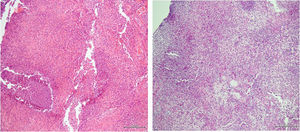
The decision to perform trans-bronchial biopsies of the lung parenchymal consolidations with rigid bronchoscope was thus taken with patient under sedation after intubation; histology is reported in Fig. 3. After the procedure, a steroid therapy with 1.5mg/kg of methylprednisolone was started and a rapid clinical improvement with a parallel resolution of the respiratory failure was observed over the following days. In agreement with the infectious diseases consultant, the therapy with doxycycline and hydroxychloroquine was started. Finally, the patient was discharged without any oxygen supplementation and steroid therapy was prolonged and gradually tapered during follow-up.
The clinical manifestation of Whipple's disease involving lung parenchyma in our patient follows other very rare cases in the past literature.4 As for the other manifestations involving different sites,2 diagnosis is confirmed at the biopsy stain both by finding the pathogen with PCR method and with the PAS positivity.1
However, the case here reported was atypical: first, we did not find PCR positivity for TW in the biopsy (despite the PAS positivity); second, patient clinically improved once systemic steroid therapy was instituted, although steroids are not a cornerstone of disease treatment.5 Indeed, since then, patient continued to deteriorate and lung infiltrates continued to worsen despite antibiotic treatment with cephalosporins, an effective first-line therapy in severe Whipple's disease.1
In the clinical history of this patient we have found that he had a dermatological manifestation probably caused by an immune reconstitution syndrome after starting the therapy for Whipple's disease. The immune reconstitution syndrome in Whipple disease is extremely rare but reported in the literature6 with a positive PAS staining, but negative PCR for TW, probably due to the previous clearance of the pathogen with antibiotic therapy.7 That was exactly what happened in our patient with lung involvement. Probably, steroid therapy might have played a role in limiting immune reconstitution. In addition, due to his hematological pathology, this patient was exposed to another risk of a immune-mediated complication i.e. the alteration of the monocytes function connected to an abnormal phagocytosis and proliferation of TW.8 The fluctuation in the number of white blood cells that we observed during admission could have led to the development of an aberrant response to antibiotics and the elimination of the pathogen.6 At discharge, specific therapy for Whipple's disease was continued because of the risk of relapse, of worsening when systemic steroids or other immunosuppressants are administered, and of manifestations due to the immune reconstitution when steroid therapy is suspended rapidly.1
To the best of our knowledge, this specific manifestation involving the lung has not been previously described and it sheds light on whether steroid therapy could be added in some cases of Whipple's disease to avoid the risk of further complications, such as immune-reconstitution syndrome.
FundingThe authors declare that no funding was received for this paper.
Consent to publish dataInformed consent to publish data was obtained by the patient.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to thank Professional Editor Colin Woodham for language editing.