Post-tuberculosis lung disease (PTLD), as other chronic respiratory disorders, may have infectious complications; some of them can be prevented with vaccinations. So far, no document has discussed the potential role of vaccination in PTLD. Therefore, the objective of this review was to describe vaccination recommendations to prevent infections potentially capable of complicating PTLD.
Materials and methodsA non-systematic review of the literature was conducted. The following keywords were used: tuberculosis, vaccination, vaccines and PTLD. PubMed/MEDLINE and Embase were used as the search engine, focusing on English-language literature only.
ResultsWe identified 9 vaccines potentially useful in PTLD. Influenza, pneumococcal and anti-COVID-19 vaccinations should be recommended. Patients with PTLD can also benefit from vaccination against shingles. Vaccination against pertussis is mainly relevant during childhood. Diphtheria, tetanus and measles vaccination are recommended for general population and should be considered in patients with PTLD not previously vaccinated. Tdap (Tetanus, diphtheria, and pertussis) booster should be repeated in every adult every ten years. Vaccination against BCG retains its importance during early childhood in countries where TB is endemic.
ConclusionsVaccination deserves to be considered among the strategies to prevent and/or mitigate PTLD complications. Further evidence is necessary to better understand which vaccines have the greatest impact and cost-benefit.
Tuberculosis (TB) is a public health priority annually affecting 10 million people, responsible for 1.6 million deaths in 2021,1 currently, the second leading infectious killer after COVID-19.2,3 Due to the complex interaction between Mycobacterium tuberculosis, host genetic factors, and immune response, 13 to 96% of patients with pulmonary TB suffer from post-tuberculosis lung disease (PTLD) after completing treatment.4,5 The World Health Organization (WHO) has estimated that from 2000 to 2020, nearly 66 million people survived tuberculosis; more than half still suffering from PLTD.2 Thus, PTLD is an important cause of chronic lung disease which was ignored for the past fifty years.6
PTLD, defined in 2019 as “evidence of chronic respiratory abnormality, with or without symptoms, attributable at least in part to previous pulmonary tuberculosis”,3,7,8 is a chronic respiratory disorder causing abnormalities such as bronchiectasis,9 and obstructive/restrictive lung disease affecting, with potential ongoing inflammation and associated tissue damage, the different components of the lungs (large and small airways, lung parenchyma, pulmonary vasculature, and pleura).3,10
Pathological features of PTLD range from modest signs and symptoms to severe dysfunction sustained by cavitation, fibrosis (both mild and severe), bronchiectasis, small-airway disease, airway stenosis, fibrosing mediastinitis, fibrothorax, and bronchopleural fistulas.3,11-15
As a result, PTLD patients often report residual cough, weakness, dyspnea, difficulties in climbing stairs (and performing other exercises) or managing every-day or work activities, which affect their quality of life (QoL) and increase the risk of death,8,16-18 while increasing the risk of recurrent TB.7
Heterogeneity in lung damage might be related to differentiation in genes that code or regulate host immune responses, host-pathogen interactions, and various immunological events.11 Potential risk factors for increasing the severity of the chronic condition PTLD are smoking (including passive), exposure to dust and biomass fuel as well as concomitant respiratory tract infections.6,13,14,19-21 HIV co-infection (depending on immunological status), Mycobacterium tuberculosis virulence, delays in diagnosis and start of appropriate anti-TB treatment as well as genetic variability of the patient could also be involved.5,11,18,22
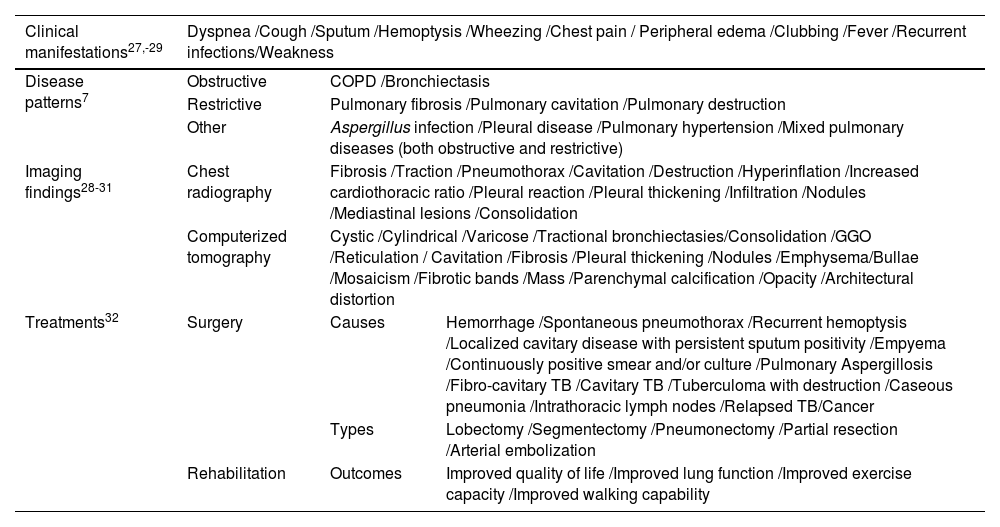
Although no evidence-based international guidelines for the management of PTLD are available7, a recent consensus document summarized the Clinical Standards for PTLD assessment, management and rehabilitation (Table 1).4,9,23
Clinical manifestations, associated-disease patterns, imaging findings, and treatment strategies of post-tuberculosis lung disease.
| Clinical manifestations27,-29 | Dyspnea /Cough /Sputum /Hemoptysis /Wheezing /Chest pain / Peripheral edema /Clubbing /Fever /Recurrent infections/Weakness | ||
|---|---|---|---|
| Disease patterns7 | Obstructive | COPD /Bronchiectasis | |
| Restrictive | Pulmonary fibrosis /Pulmonary cavitation /Pulmonary destruction | ||
| Other | Aspergillus infection /Pleural disease /Pulmonary hypertension /Mixed pulmonary diseases (both obstructive and restrictive) | ||
| Imaging findings28-31 | Chest radiography | Fibrosis /Traction /Pneumothorax /Cavitation /Destruction /Hyperinflation /Increased cardiothoracic ratio /Pleural reaction /Pleural thickening /Infiltration /Nodules /Mediastinal lesions /Consolidation | |
| Computerized tomography | Cystic /Cylindrical /Varicose /Tractional bronchiectasies/Consolidation /GGO /Reticulation / Cavitation /Fibrosis /Pleural thickening /Nodules /Emphysema/Bullae /Mosaicism /Fibrotic bands /Mass /Parenchymal calcification /Opacity /Architectural distortion | ||
| Treatments32 | Surgery | Causes | Hemorrhage /Spontaneous pneumothorax /Recurrent hemoptysis /Localized cavitary disease with persistent sputum positivity /Empyema /Continuously positive smear and/or culture /Pulmonary Aspergillosis /Fibro-cavitary TB /Cavitary TB /Tuberculoma with destruction /Caseous pneumonia /Intrathoracic lymph nodes /Relapsed TB/Cancer |
| Types | Lobectomy /Segmentectomy /Pneumonectomy /Partial resection /Arterial embolization | ||
| Rehabilitation | Outcomes | Improved quality of life /Improved lung function /Improved exercise capacity /Improved walking capability | |
Legend: COPD: chronic obstructive pulmonary disease; GGO: ground glass opacity; TB, tuberculosis.
Treatment and rehabilitation suitable for other chronic pulmonary diseases (chronic obstructive pulmonary disease or COPD, asthma, bronchiectasis among others) have been recommended for PTLD as well.4,9,23,24
Among the interventions discussed to prevent or mitigate the effects of PTLD smoking cessation, pulmonary rehabilitation, as well as vaccination, have been mentioned.25
In fact, PTLD as other chronic respiratory disorders may be complicated by viral, bacterial and fungal diseases as well as non-tuberculous mycobacteria (NTM); some of them can be prevented with existing vaccinations.26
So far, no document has discussed the potential role of vaccination in preventing or mitigating the effects of PTLD.
In this review we describe the potential role of the existing vaccinations to prevent the commonest infections potentially able to further complicate PTLD.
MethodsA non-systematic review of the literature was conducted by the members of the writing committee (including clinicians, public health and methodology experts), so as to include useful core references that may help the reader better understand the topics covered. The following keywords were used, without any time limitation: ‘tuberculosis’, ‘vaccination’, ‘vaccines’ and ‘PTLD’. The search query used the words ‘vaccination’ or ‘vaccines’ in combination with either ‘tuberculosis’ or ‘PTLD’. PubMed/MEDLINE and Embase were used as the search engine, focusing on the English-language literature only.
The records were found through database searching and merged; duplicates were removed using EndNote X7 (Thomson Reuters, Toronto, ON, Canada). Two reviewers (MMZ, ZT) independently screened the records by title/abstract and full text to include those related to the study objectives. If disagreement arose a third reviewer was asked to provide own input (MJN).
All authors, gave their input in writing specific sections of the manuscript and revising the full paper. Four versions of the manuscript were revised by the writing committee, and the final version was approved by consensus (100% agreement).
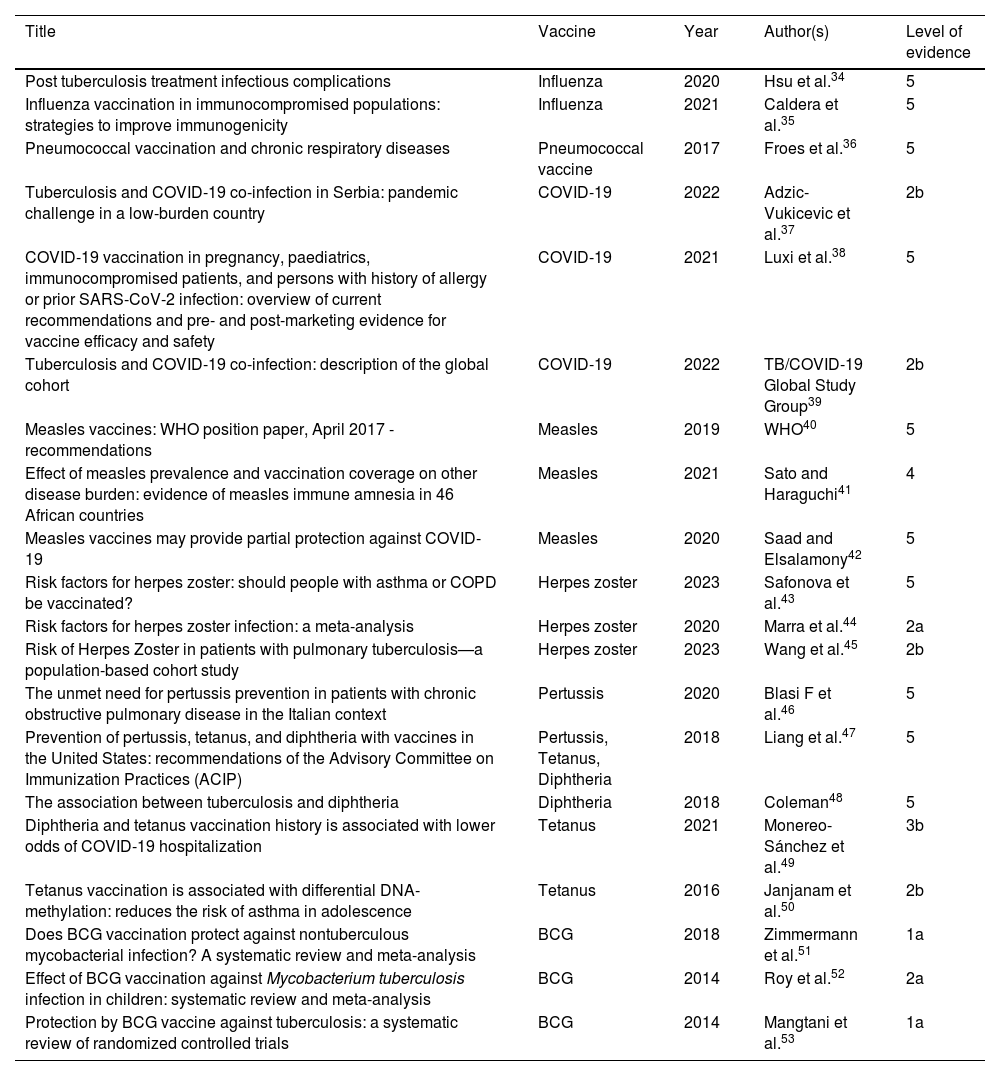
The level of evidence of the articles used in this study regarding recommendations for vaccination in PTLD patients or others is available in Table 2.33
Level of evidence on vaccination in PTLD patients and other conditions, in other chronic respiratory conditions and in the general population.
| Title | Vaccine | Year | Author(s) | Level of evidence |
|---|---|---|---|---|
| Post tuberculosis treatment infectious complications | Influenza | 2020 | Hsu et al.34 | 5 |
| Influenza vaccination in immunocompromised populations: strategies to improve immunogenicity | Influenza | 2021 | Caldera et al.35 | 5 |
| Pneumococcal vaccination and chronic respiratory diseases | Pneumococcal vaccine | 2017 | Froes et al.36 | 5 |
| Tuberculosis and COVID-19 co-infection in Serbia: pandemic challenge in a low-burden country | COVID-19 | 2022 | Adzic-Vukicevic et al.37 | 2b |
| COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: overview of current recommendations and pre- and post-marketing evidence for vaccine efficacy and safety | COVID-19 | 2021 | Luxi et al.38 | 5 |
| Tuberculosis and COVID-19 co-infection: description of the global cohort | COVID-19 | 2022 | TB/COVID-19 Global Study Group39 | 2b |
| Measles vaccines: WHO position paper, April 2017 - recommendations | Measles | 2019 | WHO40 | 5 |
| Effect of measles prevalence and vaccination coverage on other disease burden: evidence of measles immune amnesia in 46 African countries | Measles | 2021 | Sato and Haraguchi41 | 4 |
| Measles vaccines may provide partial protection against COVID-19 | Measles | 2020 | Saad and Elsalamony42 | 5 |
| Risk factors for herpes zoster: should people with asthma or COPD be vaccinated? | Herpes zoster | 2023 | Safonova et al.43 | 5 |
| Risk factors for herpes zoster infection: a meta-analysis | Herpes zoster | 2020 | Marra et al.44 | 2a |
| Risk of Herpes Zoster in patients with pulmonary tuberculosis—a population-based cohort study | Herpes zoster | 2023 | Wang et al.45 | 2b |
| The unmet need for pertussis prevention in patients with chronic obstructive pulmonary disease in the Italian context | Pertussis | 2020 | Blasi F et al.46 | 5 |
| Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) | Pertussis, Tetanus, Diphtheria | 2018 | Liang et al.47 | 5 |
| The association between tuberculosis and diphtheria | Diphtheria | 2018 | Coleman48 | 5 |
| Diphtheria and tetanus vaccination history is associated with lower odds of COVID-19 hospitalization | Tetanus | 2021 | Monereo-Sánchez et al.49 | 3b |
| Tetanus vaccination is associated with differential DNA-methylation: reduces the risk of asthma in adolescence | Tetanus | 2016 | Janjanam et al.50 | 2b |
| Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis | BCG | 2018 | Zimmermann et al.51 | 1a |
| Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis | BCG | 2014 | Roy et al.52 | 2a |
| Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials | BCG | 2014 | Mangtani et al.53 | 1a |
Legend: PTLD: post-tuberculosis lung disease; TB: tuberculosis; MDR: multidrug-resistant; WHO: World Health Organization; BCG: Bacillus Calmette–Guérin.
Level of evidence33 1a: systematic review of (homogeneous) randomized controlled trials; 1b: individual randomized controlled trials (with narrow confidence intervals); 2a: systematic review of (homogeneous) cohort studies of "exposed" and "unexposed" subjects; 2b: individual cohort study / low-quality randomized control studies; 3a: systematic review of (homogeneous) case-control studies; 3b: individual case-control studies; 4: case series, low-quality cohort or case-control studies; 5: expert opinions based on non-systematic reviews of results or mechanistic studies.
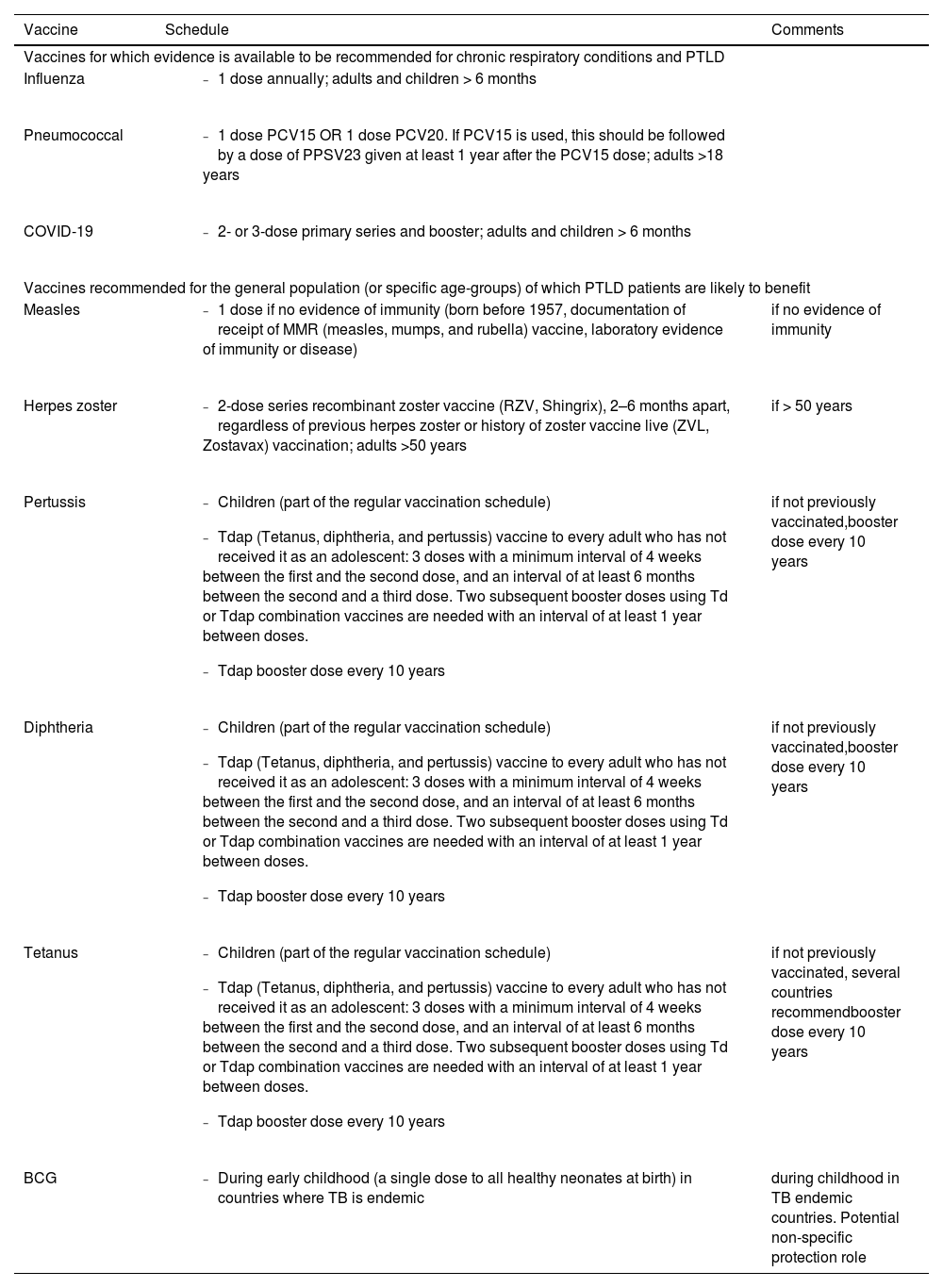
A summary of these vaccines is presented in Table 3 (in order of priority) with the recommended indications and schedule.
Suggested prioritization and schedule for vaccination in subjects with PTLD.
Legend: PTLD: post-tuberculosis lung disease; PCV15: 15-valent pneumococcal conjugate vaccine; PCV20: 20-valent pneumococcal conjugate vaccine; PPSV23: 23-valent pneumococcal polysaccharide vaccine.
Influenza is a disease caused by a group of viruses known for various epidemics they caused throughout history. Epidemics usually occur during winter (seasonal outbreaks). However, sporadic pandemics may happen in all seasons based on geography and climate. Influenza virus can strike various organs, and its symptoms emerge as a fever associated with a spectrum of systemic and respiratory symptoms.54 The first human influenza virus was isolated about 90 years ago, in 1933, which led to the production of the first live attenuated influenza vaccine. Later the first inactivated vaccine, a monovalent vaccine against type-A influenza, was produced. Following that, bivalent, trivalent, and even quadrivalent vaccines against different strains of type-A and type-B influenza virus were developed. The influenza virus mutates quickly (antigenic shift and drift). This high-speed mutation helps the virus to reshape as novel strains, leading to lower vaccine efficacy. Since 1973 WHO has published annual instructions on vaccine composition according to the circulating strains in each specific period.55
Both inactivated and live-attenuated vaccines activate the innate and adaptive immune systems. This leads to the secretion of inflammatory cytokines, interleukins, interferons, and the production of immunoglobulins (IgA, IgG1, and IgM).56 Immunocompromised patients, such as patients with HIV infection, are more vulnerable to influenza complications, putting them at higher risk of severe morbidity and mortality. WHO has recommended annual influenza vaccination for these patients; however, effective vaccination is challenging due to their incompetent immune systems.35 Patients affected by chronic respiratory conditions are more prone to suffer from influenza complications or influenza can exacerbate their pulmonary disease.57-59 United States (US) Centers for Disease Control and Prevention (CDC) describes common side effects of flu vaccination as headache, fever, nausea, myalgia and redness of the injection site. However, severe allergic reactions can occasionally occur.60 According to most international guidelines, vaccinating patients with chronic respiratory diseases against influenza is recommended, particularly those over 65 years of age. Since influenza and chronic lung conditions including PTLD can lead to higher morbidity and mortality, seasonal influenza vaccination should be recommended in PTLD patients34 (Table 3, with priority criteria for vaccination).
Pneumococcal diseasePneumococcal disease can be categorized into either invasive or non-invasive disease. Invasive disease happens when Streptococcus pneumoniae is isolated from a normally sterile site. This includes pneumococcal meningitis, bacteremic pneumococcal pneumonia, and pneumococcal bacteremia without a primary focus. On the other hand, non-invasive diseases can be divided into otitis media, sinusitis, and community-acquired pneumonia.61Streptococcus pneumoniae was first discovered in 1881. The first trials to produce a whole-cell vaccine were largely unsuccessful which brought vaccine development to a halt for a relatively long time.
Moreover, the success of penicillin led to the loss of enthusiasm for pneumococcal vaccines. Eventually, in 1977, the first 14-valent purified polysaccharide vaccine was approved for public use. Later in 1983, the vaccine-based immunization expanded to 23 capsular polysaccharides. A 7-valent conjugate vaccine was licensed in 2000 to be used in children under two years of age as their immune system response to polysaccharide antigens is relatively low.62,63
Vaccine antigens induce B cells to produce antibodies (IgM, IgG2, IgG1). This humoral immunity against pneumococcal capsule is the core mechanism of immunity. However, cell-mediated immunity plays its role by augmenting B-cell responses.63 According to CDC, common side effects of vaccination are redness and tenderness of the injection site, fever, loss of appetite, irritability, fatigue, headache, myalgia and chills. These conditions usually resolve within two days.64
A present, there are two kinds of pneumococcal vaccines available:
- •
Pneumococcal conjugate vaccines (PCV13, PCV15, and PCV20)
- •
Pneumococcal polysaccharide vaccine (PPSV23)
Evidence suggests that pneumococcal vaccination can prevent community-acquired pneumonia in patients with chronic respiratory diseases.36 Thus, it is expected that patients with PTLD, like patients affected by other chronic respiratory diseases, can benefit from pneumococcal vaccination.
CDC recommends pneumococcal vaccination (either PCV13 or PCV15) for all children younger than 2 years of age and for children 2 through to 4 years old who are unvaccinated or received an incomplete pneumococcal vaccine series.65
For adults above 65 years of age, conjugate vaccine (PCV 15) followed by polysaccharide vaccine (PPSV23) one year later is recommended, to stimulate a more vigorous immunity. In cases of certain medical conditions, such as chronic lung disease, CDC recommends conjugate vaccination (PCV 15) followed by polysaccharide vaccine (PPSV23) one year later both in children and adults. Where the new conjugate vaccine (PCV20) is used, the polysaccharide vaccine PPSV23 is not indicated.65
Vaccination for pneumococcal disease represents one of the most important preventive strategies in subjects with COPD and should be prioritized for individuals with PTLD (Table 3).
COVID-19Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Patients with SARS-CoV-2 infection may present various clinical symptoms. These mainly include cough, fever, fatigue, hyposmia, headache, nausea, and diarrhea. As of February 12, 2023, more than 755 million cases were confirmed, and more than 6.8 million deaths of COVID-19 reported to WHO globally.66,67 According to the records, it appears that the COVID-19 outbreak started on December 12, 2019, in Wuhan, China, and attempts to discover a vaccine started soon after that.68
As of today, numerous vaccines are produced against COVID-19. These vaccines are categorized as an inactivated, viral vector, live attenuated, RNA and DNA, and virus-like particle (VLP) vaccines.69 According to CDC, possible side effects of vaccination include fatigue, headache, myalgia, chills, fever, nausea and redness of the injection site. Each type of COVID-19 vaccine may have specific side effects.70
Evidence shows that COPD patients have worse outcomes from COVID-19 due to multiple biological mechanisms including micro-thrombosis, the effects of intrapulmonary shunting and secondary bacterial infection.71 For these reasons COPD patients should be prioritized for COVID-19 vaccination.72 Adzic-Vukicevic et al. conducted a retrospective analysis on TB and COVID-19 co-infection. They concluded that vaccination against SARS-CoV-2 may be beneficial in patients with current or past TB disease, including those suffering from PTLD.37,39 Given COVID-19 impacts on both patients with PTLD and TB, its use has already been recommended as a priority in these settings.73-77
Since immunocompromised patients (patients with malignancy, organ transplant recipients, patients with HIV, etc.) are at higher risk of severe SARS-CoV-2 infection, they are prioritized for COVID-19 vaccination38 (Table 3).
PertussisPertussis (also known as whooping cough) is a highly contagious vaccine-preventable disease. Symptoms of pertussis start with rhinorrhea, lacrimation, congestion of respiratory mucosa, conjunctival hyperemia, sore throat, and coughs, which deteriorate as the disease progresses.78 Historically, pertussis disease was recognized centuries ago. However, its causative agent, Bordetella pertussis, was discovered in 1906, leading to the invention of a whole-cell pertussis vaccine in 1914. This significantly decreased the morbidity and mortality from pertussis.79
Two types of pertussis vaccine are categorized as a whole-cell vaccine (wP) and acellular vaccine (aP), respectively. As its name suggests, the whole-cell vaccine contains the entire inactivated cell of Bordetella pertussis, while the acellular vaccine contains only some specific antigens. Whole-cell vaccines are associated with more side effects in comparison to acellular vaccines; however, they are more efficacious.78 According to CDC, common side effects of the combined diphtheria, tetanus and acellular pertussis (DTaP) vaccine are swelling of the injection site, fever, irritability, fatigue, vomiting and loss of appetite which are mild to moderate in severity and can last 1–3 days.80 Whole-cell pertussis vaccine induces immunity mainly through Th1 and Th17-mediated responses by activating CD4+ T cells. Acellular vaccines, in contrast, primarily induce Th2-mediated responses.81 Vaccination during infancy and early childhood induces and maintains immunity against pertussis for several years. However, immunity may wane in older age leading to disease resurgence. Thus, many researchers have mentioned the need to modify immunization schedules to reduce circulation within families and thus guarantee protection for young children and adults at risk of complications.82
Emerging data shows that individuals with COPD are at high risk of contracting pertussis. Furthermore, those who develop pertussis could experience exacerbation of their pre-existent COPD and further susceptibility to other infections, leading to increased rate of hospitalization and direct medical costs compared to controls (patients without a diagnosis of COPD).46,83 Patients suffering from PTLD share several clinical and pathological characteristics with chronic lung disease such as COPD, asthma, bronchiectasis.4,9,23,24 Thus, pertussis vaccination is expected to have a positive impact among them, too.46,83
To prevent pertussis, US CDC recommends diphtheria, tetanus, and pertussis (DTaP) vaccines in children below 7 years of age, and Tdap (tetanus, diphtheria, and pertussis vaccine) for children 7 years and older, adolescents, and adults. Pregnant people should get a dose of Tdap during every pregnancy, preferably during the early part of the third trimester, to help protect the newborn from pertussis, as infants are most at risk of severe, life-threatening complications from pertussis. Adults who have never received Tdap should get a dose of Tdap, and this also applies to patients with chronic respiratory disease. Also, adults should receive a booster dose of either Tdap every 10 years84 (Table 3).
Herpes zosterVaricella zoster virus (VZV) is a type of human herpesvirus that can cause two different diseases. These are primary infection (also known as chickenpox) and reactivation of latent infection (herpes zoster or shingles). Shingles emerges as a vesicular rash that extends along dermatomes. The first varicella vaccine was developed in Japan in 1974 as a live-attenuated vaccine and was used to reduce morbidity and mortality among immunocompromised children.85 ZVL, a live attenuated vaccine, was the first FDA-approved vaccine to be used against herpes zoster in 2006. Later in 2017, FDA approved RZV, an adjuvanted recombinant zoster vaccine, for adults over 50 years of age. In 2021 FDA further approved RZV for adults aged ≥ 18 years with increased risk for herpes zoster due to immunosuppression or immunodeficiency.43 The latter vaccine, SHINGRIX, stimulates cytokine production and promotes CD4+ T-cell responses and antigen-specific antibody production.86 Side effects of Shingrix include tenderness and redness of the injection site, fatigue, myalgia, headache, fever, chills and nausea. Symptoms usually resolve within 2–3 days.87 A recent meta-analysis revealed that COPD increases the risk of herpes zoster by 41%.44 Moreover, a COPD patient is more likely to develop post-herpetic neuralgia following herpes zoster.43 CDC suggests routine use of shingles vaccine in patients with lung diseases.88 A recent cohort study concluded that pulmonary TB, as a stressor, significantly increases the risk of herpes zoster occurrence.45 Thus, it seems that patients with PTLD, similarly to patients with COPD, can benefit from vaccination against shingles (Table 3).
DiphtheriaDiphtheria is an acute respiratory infection caused by toxin-producing bacteria from the Corynebacterium genus. The discovery of diphtheria antitoxin, penicillin, toxoid vaccine, and mass vaccination significantly reduced morbidity and mortality from diphtheria. Thus, it has recently escaped researchers' attention.89 There were diphtheria epidemics centuries before Pierre Bretonneau named it in 1826. In 1914 a safe mixture of toxins and antitoxins was used for immunization. Later diphtheria toxoid was developed, and mass immunization of schoolchildren began. Since 1974 WHO has included diphtheria toxoids along with tetanus and pertussis vaccines (DTP) in childhood vaccination programmes.90
Diphtheria toxoid-based vaccine is a purified formulation of inactivated diphtheria toxin. This toxoid induces immune responses to produce antibodies against toxins.91 Although diphtheria is controlled in the majority of countries, many outbreaks were recorded last century. Therefore, the risk of potential resurgence and seizures should be considered.90 It is recommended that individuals living in areas of low endemicity should receive a booster dose of diphtheria toxin every ten years after the first series of vaccination, as diphtheria immunity wanes throughout ones life.47 Based on historical reports, in a tuberculosis-diphtheria co-infection, the severity of either disease intensifies. Also, a severe diphtheria infection may “re-awake” an earlier tuberculosis infection.48 Thus, diphtheria vaccination should be considered in not previously vaccinated PTLD patients, as in general population (Table 3).
TetanusTetanus disease, previously known as lockjaw, is caused by a gram-positive, obligatory anaerobic bacteria called Clostridium tetani, which is often found in soil and feces in the environment and can turn into a progressive, fatal condition.92,93 Tetanus is typically caused by these bacteria entering the body through wounds, cuts, abrasions, or punctures in cutaneous or mucosal barriers. Also, in rare cases, surgical procedures, intravenous drug injections, and animal/insect bites may lead to tetanus.92 Once entered, Clostridium tetani migrates into the nervous system, where it begins to produce tetanospasmin, which usually causes muscle rigidity, autonomic system dysfunction, and muscular spasms, and tetanolysin, the exact function of which is not fully understood.94 Although global immunization with the tetanus toxoid vaccine since 1940 has led to a dramatic reduction in tetanus infection and mortality rates, tetanus still leads to thousands of deaths worldwide, especially in low and middle-income countries where the immunization rate is low. In 2015, more than 56,700 tetanus-related deaths occurred, with Asia and Sub-Saharan Africa accounting for 45% and 44% of mortalities, respectively.95 Since not having immunization from tetanus remains a significant risk factor for tetanus development and mortality, many communities still recommend and perform tetanus vaccination for the whole population.95
The first administration of tetanus toxoid as a vaccine tith the purpose of tetanus prevention goes back to 1924. To obtain the toxoid vaccine, Clostridium tetani is cultured in a liquid media, and then, the toxin is obtained, purified, and becomes inactivate using formaldehyde and is further formulated with mineral salts, such as aluminum and calcium.96 Till 1938, several advances were made in developing a safer and more effective tetanus toxoid vaccine. Since 1940, the tetanus vaccine has become widely used worldwide.97 Leading to a remarkable reduction in tetanus cases since its emergence, the tetanus toxoid is considered a safe and effective way for tetanus prevention and is a vital part of routine vaccination programmes in almost all countries, usually injected along with diphtheria and pertussis vaccines. Based on WHO recommendations,98 administration of the first dose of tetanus toxoid should be initiated in infancy at six weeks of age, and the second dose must be injected at least four weeks apart. At six months, the third dose should be given to complete the primary series. In addition to these three primary doses, three booster doses should also be administered, which help to make life-long immunity protection against infection with Clostridium tetani. The first booster dose is recommended to be injected at 1–2 years of age, and the second and third booster doses should be administered at least four years apart.98
The beneficial effects of the tetanus vaccine come mainly through the induction of immune response. Once injected, the tetanus toxin activates T helper cells and B cells, which further work together to produce and secrete cytokines that provide immune protection against the natural toxins of Clostridium tetani.99
Since the majority of the immune response against Clostridium tetani is based on antibodies that wane through time, the administration of periodic booster doses of tetanus toxoid is recommended to provide lifelong protection against the disease.100
However, uncertainty exists about if and when to provide booster doses in adults. Some countries and organizations, such as the United Kingdom and the WHO do not recommend additional dosage after adolescence (but they do not recommend against it), while in other countries the recommendation is to provide a booster dose of Td (tetanus, diphtheria) or Tdap every 10 years or more.84,101-105
Although scarce, a few studies revealed the non-specific effects of the tetanus vaccine in various infectious diseases in addition to tetanus. Recently, a growing body of literature showed a possible association between the history of tetanus vaccination and the risk and/or severity of COVID-19 infection. Regarding this issue, Monereo-Sánchez et al. demonstrated that individuals with a history of receiving tetanus vaccine during the last ten years were at a lower risk of developing severe COVID-19.49 Moreover, some evidence indicating the protective effect of tetanus vaccination against developing some respiratory disorders, such as asthma, in adolescence has been found.50 Although the association between tetanus vaccination and TB or PTLD has not been well studied yet, in accordance with previous studies regarding the heterologous effects of tetanus toxoid vaccine on respiratory infections and diseases, the vaccine might be able to exert some beneficial effects on TB or its associated pulmonary disorders (Table 3). However, more evidence on this is required.
MeaslesMeasles, characterized and annotated by Thomas Sydenham in the 17th century, is a highly contagious infectious disease caused by the ribonucleic acid (RNA) respiratory measles virus.106,107 Typically, measles presents with progressive fever, maculopapular rash initiating from the face and spreading to the whole body surface, coryza symptoms, and Koplik spots, which are pathognomonic of the disease.108 In addition to the abovementioned typical symptoms, measles can be accompanied and complicated by severe respiratory, gastrointestinal, neurological, and ocular manifestations.106,109,110 Being highly contagious, the measles virus led to several outbreaks in many parts of the world before the development and broad implementation of the vaccine.111
The measles vaccine was first developed in 1954 when Thomas Chalmers Peebles and John Franklin Enders separated the measles virus from the nasopharyngeal sample of a child and cultured the virus on a culture consisting of primary human kidney cells.112,113 A live attenuated vaccine was developed by further passaging the isolated virus in renal, amnion, and embryo fibroblast cells.112 The protection against measles infection is mostly caused by the vaccine-induced activation of both humoral and cellular immune responses.107 Following measles vaccination, T cells are activated, which then contribute to the production of antibodies by B cells, especially those against the H protein of the measles virus. These B cell-produced antibodies usually provide the vaccine recipient with long-lasting protection against measles infection.114,115
This live attenuated vaccine was very successful in reducing the incidence of measles infection and associated mortality. Nowadays, based on the WHO recommendations, all susceptible individuals should receive two doses of the measles vaccine.40 Mainly, the measles vaccine is administered in combination with the mumps and rubella vaccines (MMR). It has been stated that reaching a vaccine coverage of 95% or higher can result in measles elimination in any region.40 Thanks to the contribution of the measles vaccine, a 79% reduction in measles cases and a 94% reduction in measles-related mortality occurred from 2000 to 2020.116 However, despite a high vaccination rate in most countries, measles can still cause outbreaks in some regions.108 According to CDC, side effects of vaccination with the MMR vaccine may include tenderness and redness of the injection site, fever, mild rash and temporary pain and stiffness of the joints.117
In addition, to provide outstanding protection against measles infection, some studies have previously shown that the measles vaccine might be helpful in reducing the burden of some other diseases. For instance, Sato et al. showed that measles vaccination was associated with not only a reduction in measles cases and deaths but also a lower prevalence and mortality of diarrhea, low respiratory infections, malaria, meningitis, and TB.41. More recently, a few studies suggested that a history of vaccination with the measles vaccine might lead to a lower rate of COVID-19 infection and mortality, in part due to the activation of the immune system and the non-specific effects of the vaccine on all-cause mortality. However, the potential association is not well studied yet and needs further evaluation.42 Although further evidence is necessary, a rationale exists to consider this vaccination in unvaccinated individuals suffering from chronic respiratory diseases (and for PTLD), as in the general population at large (Table 3).
BCGThe first and early research activities of Albert Calmette and Camille Guérin for developing a vaccine against Mycobacterium tuberculosis were conducted in early 1900 at the Pasteur, Lille, France.118 Historically, to generate an effective vaccine, the virulent Mycobacterium bovis pathogen was passaged by Calmette and Guérin on a medium consisting of glycerin, potato, and ox bile.119 After almost 230 passages since 1908, finally, an attenuated form of Mycobacterium bovis was introduced in 1919 which was unable to cause tuberculosis infection in animals and was named Mycobacterium bovis bacille Calmette Guѐrin (BCG). In 1921, the BCG vaccine was first tested in the human population.120 Over these 102 years, BCG has been widely used worldwide as the sole effective vaccine against tuberculosis and was associated with promising results regarding the reduction of tuberculosis infection and mortality.52,120 Nowadays, BCG is considered a primary part of routine vaccination programmes in many countries where TB is endemic. Some countries with a lower prevalence of TB also administer the BCG vaccine to those who are at a high risk of developing and transmitting TB, people like healthcare workers.121
Although the exact mechanisms through which the BCG vaccine exerts its beneficial effects against Mycobacterium tuberculosis are not fully figured out, it is widely believed that the vaccine affects the host immune system as it builds up a robust and appropriate immune response against the pathogen.120 It has been shown that BCG could induce both innate and adaptive immune systems as well alter the immune regulatory responses and factors. Once injected, BCG can induce monocytes, macrophages, neutrophils, and dendritic cells to produce cytokines, such as IFN-γ, and further activate adaptive immune system components, including CD4+ and CD8+ T cells to secret IL-2, TNF-α, and IL-10.122-126 Although it seems that BCG provides its beneficial effects mostly via activation of the immune system, the exact underlying mechanisms are yet to be understood. Side effects of BCG vaccination include pain and discharge from the injection site, fever, headache and axillary lymphadenopathy. Severe complications such as abscess, osteomyelitis and disseminated BCGitis are rare.127
Although the BCG vaccine was primarily developed to combat TB, previous studies have found that BCG vaccination might also have beneficial effects in the prevention of infectious diseases other than TB; proposedly due to its power to activate the immune system response.128,129 A systematic review and meta-analysis carried out by Zimmermann et al. revealed that the BCG vaccine had a more protective effect against nontuberculous mycobacteria in children who had previously received vaccination than in those without a history of BCG vaccination at birth or childhood.51 Recently, several studies evaluated the protective effect of the BCG vaccine in COVID-19 infection development;130-132 however, the results are conflicting. A systematic review and meta-analysis conducted by Li et al. demonstrated that individuals who were previously vaccinated with BCG were at a 0.61-fold lower risk of developing infection with SARS-CoV-2 virus.133 In contrast, Wen et al. stated that BCG vaccination had no significant effect on COVID-19 development or severity regarding hospitalization, intensive care unit admission, or mortality.134 Although a growing body of literature has revealed the beneficial off-target effects of BCG vaccine in prevention of various infectious diseases caused by infectious microbial agents other than Mycobacterium tuberculosis, such as NTM diseases, these non-specific effects need further evaluation.51 However, the efficacy of BCG vaccine in prevention of some respiratory disorders has been evaluated. For instance, Li et al. found that children with bronchiolitis who were vaccinated with BCG had a lower risk of developing bronchial asthma than those who were not BCG vaccinated.135 Despite the efficacy of immunization with BCG in preventing TB has been evaluated in numerous studies,52,53 data about the impact of BCG vaccination in prevention of PTLD and its adverse outcomes is very scarce and is yet to be understood. In summary, BCG protects against TB and probably has a non-specific role in protecting from other infections, although its role in reducing relapse or reinfection in PTLD remains to be elucidated. Therefore, vaccination against BCG retains its importance during early childhood in countries where TB is endemic, while new and more effective vaccines will be made available for programmatic use (Table 3).
Research gapsSeveral research gaps exist on the role of vaccines in managing subjects with PTLD. Below we report some of them, as a contribution to further discussion:
- •
Lack of longitudinal data from TB survivors that includes non-TB respiratory infections like pneumococcus, influenza and other lower respiratory tract pathogens.
- •
What the risk is for a PTLD person of developing Low Respiratory Tract Infections (LRTIs) compared with an age matched control.
- •
Are LRTIs of similar severity and outcome in PTLD comparable with those in the general population?
- •
Should all PTLD patients receive vaccination for influenza, pneumococcus, pertussis, Respiratory Syncytial Virus (RSV) or can PTLD patients be placed into subcategories based on risk (i.e., age, HIV status, other comorbidities/immunosuppressive concomitant medications, chest X-ray appearance)?
- •
Studies are needed to understand the role of Hemophilus Influenzae vaccination in subjects with PTLD.
- •
Further evidence is needed to understand if BCG has a real non-specific effect to protect patients with chronic respiratory conditions.
Considering the epidemiological relevance of PTLD, the role of vaccination has an important contribution to prevention of complications and/or mitigation of their effects. Immunizing PTLD patients with the available vaccines as primary immune response inducers, or booster doses has been accompanied by considerable benefits. All in all, since PTLD as a chronic respiratory condition impacts on the respiratory system, vaccinating against preventable respiratory infections could efficiently limit secondary infections and further evolution of PTLD damage. Therefore, vaccination deserves to be considered among the strategies to prevent and/or mitigate PTLD, reinforcing the existing recommendations for the general population and emphasizing the need for extended vaccine coverage for PTLD patients regardless of their age. Further evidence from longitudinal studies and modeling is necessary to better understand which vaccines have the greatest impact and cost-benefit.
Funding sourceThis work was partially funded by the “Ricerca Corrente” scheme of the Ministry of Health, Italy and by an educational grant of Glaxo Smith Kline to SBPT (Sociedade Brasileira de Pneumologia e Tisiologia).
None.
The article is part of the scientific activities of the Global Tuberculosis Network (GTN).
The authors thank Luciano Attard (Infectious Diseases Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy) for his contribution.