Abdominal obesity is associated with a risk of cardiovascular diseases, metabolic syndrome and decreased lung function. However, it is not known whether asthma control is influenced by the accumulation of adipose tissue in the various abdominal compartments.
ObjectiveTo determine associations among abdominal adiposity distribution, asthma control, lung function and cytokines in women.
Methods and designIn this cross-sectional study of asthmatic women, data on demographic variables, comorbid conditions, disease history, anthropometric and spirometric measurements were collected. Subcutaneous (SAT) and visceral (VAT) adipose tissues were measured by ultrasound, and the steatosis level was obtained. Asthma control was assessed according to Global Initiative for Asthma (GINA) criteria. Atopy was defined on the basis of allergen-specific Immunoglobulin E and/or skin prick testing. Cytokine levels were determined using enzyme-linked immunosorbant assays (ELISAs).
ResultsEighty-three asthmatic women were included, 37% of whom had uncontrolled asthma. After controlling for variables, a negative association between asthma control and VAT and the VAT/SAT ratio was observed. VAT was negatively associated with respiratory parameters after controlling for explanatory variables. In an adjusted model, body mass index (BMI) and SAT were inversely associated with the adiponectin serum level and VAT was associated with the interleukin 6 level. In conclusion, visceral obesity was negatively associated with asthma control and lung function; and positively associated with increased levels of interleukin 6 in women. We hypothesize that women should be studied as a separate group, and we suggest further studies with a control group to know if the uncontrolled asthmatic group is directly affected by visceral adipose inflammatory markers.
It was recently reported that abdominal obesity is increasing faster than overall obesity.1 Abdominal obesity is associated with cardiovascular risk, metabolic syndrome2,3 and decreased lung function.4 However to date, there is a lack of correlation between obesity related inflammatory markers and asthma.5 The precise mechanism and the significance of the association between adipokines, asthma and visceral obesity, considered to be the most inflammatory are unknown. Most studies investigating the association between asthma and obesity have considered only body mass index.6,7 However, the use of this parameter has limitations because it does not distinguish between fat mass and muscle or identify the distribution of body fat.8 We hypothesized that the accumulation of abdominal fat in the visceral compartment, which is particularly associated with increased inflammatory markers,9,10 could adversely affect lung function and worsen asthma control in women. It is possible that the greater risk and morbidity of asthma in women11,12 is in part due to the hormonal changes that occur throughout life,13 and these changes may be associated with different forms of fat accumulation, which may interfere with asthma immunoregulation and control. Therefore, we aimed to evaluate the associations among ultrasound measurements of abdominal fat, serum concentrations of inflammatory cytokines, asthma control and lung function in women.
Materials and methodsThis cross-sectional study was conducted in asthmatic women diagnosed with persistent asthma, as defined by Global Initiative for Asthma (GINA)14 criteria, using clinical data and lung function testing in an outpatient asthma clinic at the University Hospital Gaffrée and Guinle (HUGG) from August 2014 to January 2015. The subjects were older than 17 years and were routinely followed up for more than one year. Treatment with fixed doses based on GINA guidelines14 were initiated at least three months prior to the study by the same physician. All the patients under 65 years old had BMI values between 18.5 and 39.9kg/m2 (weight in kilograms divided by the square of the height in meters), whereas the BMI for those older than 65 was between 22.0 and 41.9kg/m2 and 22.0 and 41.9kg/m2.15 Subjects were excluded if they were current smokers or had smoked within the past 5 years, were pregnant or nursing, had a history of psychiatric diseases, active pulmonary disease, malignancy, immunodeficiency, autoimmune diseases, congestive heart failure, cardiovascular disease, thyroid dysfunction, were chronic users of systemic corticosteroids or immunosuppressive drugs, had previous use of immunotherapy or had experienced an airway infection or exacerbation during the four weeks preceding the study.
Data collectionDemographic data [age and education (less than or more than five years)], number of parities, duration of disease (less or more than 15 years), onset of disease (before or after the age of 12 years), onset after menopause, rhinitis symptoms, use of drugs for diabetes, hypertension or gastro-oesophageal reflux disease (GERD), use of systemic corticosteroids and emergency room visits during the year prior to the study, assessment of asthma severity and control, anthropometric measurements (weight, height, waist circumference), and pulmonary function parameters including pre-bronchodilator percent-predicted FEV1 (forced expiratory volume in one second), FVC (forced vital capacity) and the FEV1/FVC ratio were obtained.
Assessment of asthma control and severityAsthma control was defined as fully, partly controlled or not controlled according to the GINA guidelines 2012.14 In addition, asthma severity was defined based on the GINA 2002 guidelines, which divide patients into 4 categories, mild intermittent, mild, moderate and severe persistent, based on the frequency of symptoms, spirometry results and pharmacological treatment.16 Peak expiratory flow (PEF), expressed as the percentage of the predicted value based on age, sex, and height, was used to assess asthma control and severity.17
Anthropometric measuresWeight and height were measured to the nearest 0.1kg and 0.1cm, respectively, according to standard protocols.15 Waist circumference (WC) was measured to the nearest 0.1cm using a nonelastic tape measure, at the midpoint between the lower costal margin and iliac crest at the end of a normal expiration in supine position, with no clothes over the site of the measurement and with the arms extended laterally and the feet together. All measurements were performed by the same physician. BMI, waist-to-height ratio (WHtR) and waist-to-hip ratio (WHR) were calculated. The BMI value was categorized as normal, overweight or obese according to WHO (World Health Organization) criteria. Central obesity was defined according to WC, WHtR and WHR measurements. The patients younger than or older than 65 years were considered eutrophic when their BMI values were in the ranges of 18.5–24.9kg/m2 or 22–27kg/m2, overweight with BMI values in the ranges of 25–29.9kg/m2 or 27.1–32kg/m2, and obese with BMI values in the ranges of 30–39.9kg/m2 or 32.1–41.9kg/m2.16 Central obesity was defined as a waist circumference over 88cm, according to WHO criteria, as was a WHtR greater than 0.50 and a WHR greater than 0.85.15
IgE and allergy skin-prick testingSerum samples were analyzed for total and allergen-specific IgE using the Pharmacia Diagnostics ImmunoCAP 250 system (Sweden). The types of allergens for which specific IgE tests were performed included two house dust mites (Dermatophagoides pteronyssinus and Blomia tropicalis) and one fungus (Aspergillus). A concentration of specific IgE>0.10kU/L was defined as a positive test result. Allergic sensitization to 8 aeroallergens: three dust mites (D. pteronyssinus, D. farinae and B. tropicalis), two fungi (Alternaria and Aspergillus), and dander from three animals (cat, dog and cockroach), was determined in all subjects using skin-prick tests according to EAACI (European Academy of Allergology and Clinical Immunology)18 guidelines. The skin-prick test was performed by Alergolatina Laboratory (Rio de Janeiro, Brazil). Sensitization was defined as present with a wheal that was greater than 3mm and greater than that produced by the saline negative control. Atopy was defined by a positive result for any of the allergen-specific IgEs and/or skin-prick test for any of the aeroallergens listed above.
Ultrasound measurementsUltrasonographic measurements were performed according to the criteria of Diniz et al.19 by a single physician using an Ultrasound ACCUVIX-V10. The subcutaneous fat thickness was measured with a 7.5MHz linear transducer transversely positioned 1cm above the umbilical scar. For visceral fat, a 3.5MHz transducer was also positioned 1cm above the umbilical scar and was considered to be the distance between the internal surface of the abdominal rectus muscle and the posterior aortic wall in the abdominal midline. The criteria for assessing the severity of hepatic steatosis were those of Saadeh et al.,20 which divide steatosis into 3 categories: Grades I (mild), II (moderate), and III (severe).
Assessment of respiratory functionPulmonary function tests were performed according to American Thoracic Society (ATS) guidelines21 by a single physician using a Spirometer Spiron 2 (Codax Corporation; São Paulo, Brazil) to determine the pre-bronchodilator-predicted FEV1%, FVC%, and FEV1/FVC ratio. The baseline FEV1 recorded was the best of three reproducible values from acceptable curves with less than 5% differences in amplitude. We used the predicted pulmonary values of the Knudson standards.22
Biochemical assaysPeripheral blood was centrifuged, and the serum was divided into 200μl aliquots and frozen at −80°C prior to analysis. Adiponectin, interleukin 6 (IL-6), interleukin 8 (IL-8), transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α) and plasminogen activator inhibitor (PAI) levels were determined using commercially available enzyme-linked immunosorbant assay (ELISA) kits (Affymetrix eBioscience, San Diego); the detection sensitivity levels were 10pg/ml, 2pg/ml, 2pg/ml, 8pg/ml, 4pg/ml and 78pg/ml, respectively.
Statistical analysisBivariate analyses were performed using the t test for continuous variables and the chi-square test for categorical variables to investigate the relationships between clinical and demographic characteristics and asthma control. Variables showing an association with a p-value up to 0.20 in the bivariate analysis were used in regression models. In logistic regression, a dichotomous dependent variable was used for asthma control (controlled/partly controlled versus not controlled). The results were expressed as odds ratios (OR) and 95% confidence intervals. For analyses of the association of respiratory function parameters, the pre-bronchodilator predicted FEV1%, FVC% and FEV1/FVC ratio were defined as dependent variables. Adiponectin and IL-6 were also used as dependent variables in linear regression models. The level of significance was set at 5%. All analyses were performed using SPSS statistical software, version 17.0. The study was approved by the Research Ethics Committee of HUGG on June 2014.
ResultsEighty-three women with ages ranging from 18 to 82 years old with a mean age of 55.39±13.67 years, were included. A total of 37.3% of women were found to have uncontrolled asthma. Among the subjects 72.3% were overweight or obese according to BMI. Using WC, WHR and WHtR criteria, central obesity was detected in 75.9%, 72.3% and 91.6% respectively. The mean of subcutaneous abdominal fat tissue (SAT) was 2.00±0.59 and the mean for the visceral form was 4.57±2.42cm. Pearson correlation coefficients for BMI with WC (r=0.78) and visceral fat (r=0.64) were better than those for the other measures of adiposity. In addition, significant differences in the level of asthma control as a function of visceral adipose tissue (VAT) and the VAT/SAT ratio were observed. Adiponectin levels were also associated with asthma control, as were age, use of systemic corticosteroids, emergency room visits in one year, atopy, asthma severity and pulmonary parameters. There was a significant difference between asthma control and VAT and the VAT/SAT ratio after adjusting for confounders. The use of systemic corticosteroids and room emergency visits in one year were also associated with asthma control in the three models employed. These results are shown in tables in supplementary materials.
In linear regression models, we found negative associations among age, TAV, asthma control, FEV1 and CVF after controlling for BMI, rhinitis, use of systemic corticosteroids in one year, onset of asthma, atopy, disease duration and serum adiponectin (Tables 1 and 2).
Linear regression model of pre-bronchodilator-predicted FEV1% in asthmatic women; HUGG, 2014–2015.
| FEV1% predicted | |||||
|---|---|---|---|---|---|
| Independent variable | Category | Model 1aa β (95% CI) | Model 2bb β (95% CI) | Model 3cc β (95% CI) | Model 4dd β (95% CI) |
| Adipose tissue | Centimeters | −2.87 (−11.83 to 6.08) | −3.45† (−6.71 to −0.19) | −3.54 (−8.39 to 1.30) | −0.26 (−0.95 to 0.41) |
| Age | Years | −0.83† (−1.25 to −0.41) | −0.80† (−1.20 to −0.40) | −0.75† (−1.17 to −0.33) | −0.77† (−1.19 to −0.35) |
| Asthma control | Controlled/partially controlled | Baseline | Baseline | Baseline | Baseline |
| Uncontrolled | −17.62† (−28.91 to −0.62) | −14.52† (−25.41 to −3.63) | −13.82† (−25.40 to −2.24) | −17.25† (−28.28 to −6.11) | |
| Arterial hypertension | No | Baseline | Baseline | Baseline | Baseline |
| Yes | 13.71† (2.85 to 24.58) | 13.92† (3.36 to 24.48) | 13.93† (3.19 to 24.67) | 13.57† (2.73 to 24.41) | |
Controlled for BMI, rhinitis, use of systemic corticosteroids in one year, onset of asthma, atopy, disease duration, serum adiponectin.
Linear regression model of pre-bronchodilator-predicted FVC % in asthmatic women; HUGG, 2014–2015.
| FVC% predicted | |||||
|---|---|---|---|---|---|
| Independent variable | Category | Model 1aa β (95% CI) | Model 2bb β (95% CI) | Model 3cc β (95% CI) | Model 4dd β (95% CI) |
| Adipose tissue | Centimeters | −4.07 (−12.45 to 4.30) | −3.61† (−6.65 to −0.57) | −2.71 (−7.29 to 1.85) | − 0.37 (−1.01 to 0.26) |
| Age | Years | −0.64† (−1.03 to −0.24) | −0.60† (−0.97 to −0.22) | −0.56† (−0.96 to −0.17) | −0.56† (−0.95 to −0.16) |
| Asthma control | Controlled/partially controlled | Baseline | Baseline | Baseline | Baseline |
| Uncontrolled | −15.55† (−26.12 to −4.99) | −12.00† (−22.15 to −1.85) | −12.10† (−23.03 to −1.17) | −15.03† (−25.33 to −4.72) | |
| Arterial hypertension | No | Baseline | Baseline | Baseline | Baseline |
| Yes | 10.53† (0.37 to 20.69) | 10.71† (0.87 to 20.55) | 10.63† (0.50 to 20.76) | 10.33† (0.20 to 20.45) | |
Controlled for BMI, rhinitis, use of systemic corticosteroids in one year, onset of asthma, atopy, disease duration, serum adiponectin. FVC (forced vital capacity) % predicted.
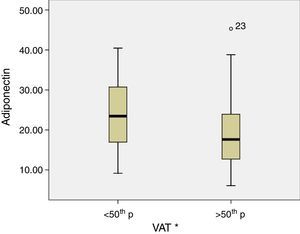
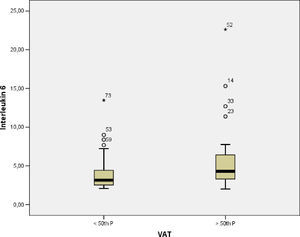
The levels of adiponectin decreased as a function of BMI (p-value=0.005), SAT (p-value=0.03) and VAT (p-value=0.04) (Fig. 1), and IL-6 levels were increased, though not significantly, as a function of TAV (p-value=0.14). To determine the association between cytokine levels and adipose tissue, linear models were fitted and adjusted using previously described covariates. In a linear regression model with adiponectin as the dependent variable, significant associations between adiponectin and BMI and SAT after adjusting for age, FEV1, arterial hypertension, diabetes, and asthma control were found. However, IL-6 was only associated with TAV after adjustment for the same covariates (Table 3).
Linear regression model of serum levels of adiponectin and interleukin-6 in asthmatic women; HUGG, 2014–2015.
| Adiponectin (ng/ml) | Interleukin-6 (pg/ml) | |||
|---|---|---|---|---|
| Model β (95% CI) | p | Model β (95% CI) | p | |
| BMI | −0.69 (−1.19 to −0.19) | 0.007 | 0.22 (−0.13 to 0.58) | 0.21 |
| SAT | −4.08 (−7.50 to −0.66) | 0.02 | 2.05 (−0.36 to 4.47) | 0.09 |
| VAT | −1.14 (−2.4 to −1.11) | 0.07 | 1.20 (0.31 to 2.09) | 0.009 |
Controlled for age, FEV1, arterial hypertension, asthma control and diabetes. BMI (body mass index), SAT (subcutaneous adipose tissue), VAT (visceral adipose tissue).
Our results show that VAT, but not anthropometric measures of central obesity, was associated with impaired asthma control after adjusting for independent variables, including BMI.
Although asthma control and central obesity have been shown in a few studies to be associated, these studies have only considered anthropometric measures and their implications are different views in relation to assessment instruments for asthma control.11,12 Conversely, abdominal subcutaneous and visceral fat deposits in asthmatic patients have been little explored.23,24
Tavasoli et al.,25 did not suggest any association of WC or WHR with asthma control, nor did the recently published Brazilian study by Barros et al.26 In contrast to our study, asthma control in the latter study was assessed using a self-administered questionnaire, the ACQ (Asthma Control Questionnaire), and only severe patients from both sexes were included. However, in a recent study of uncontrolled asthmatic patients, the authors observed an association between the worst levels of asthma control assessed by ACT (Asthma Control Test) and central obesity measures, independent of GERD, OSA (obstructive sleep apnoea) and BMI.27 Our group also recently reported a negative association between WC and asthma control in women assessed by GINA and ACQ but not with ACT results after controlling for GERD and other variables.28 The differences in these results may be due to the greater disease severity as well as the use of the fully controlled category as the comparator. In the current study, our central hypothesis was that visceral obesity is associated with poorly controlled asthma, inflammatory cytokines and decreased lung function in women. For this reason, we used GINA criteria, which are considered to be the most recommended asthma assessment tool worldwide, with a better correlation with other tools in recognizing the uncontrolled group.29 This present study also showed a significant association of the VAT/SAT ratio with worse asthma control, highlighting the possible protective factor of subcutaneous fat. Indeed, Weber et al. suggested the protective effect of abdominal and gluteal-femoral obesity in a study in which they induced metabolic syndrome with a high-fat diet in animals that were then subjected to lipectomy of abdominal subcutaneous tissue.30
We also observed a negative association of visceral fat with the pre-bronchodilator- predicted values of FEV1% and FVC%. To date, few studies have evaluated the effect of abdominal obesity on lung function or the relationship between respiratory parameters and abdominal fat deposits. Fenger et al. demonstrated that visceral abdominal fat assessed by ultrasound is associated with greater reductions in FEV1 and FVC compared with subcutaneous fat and anthropometric measures of central obesity.23 We also observed a borderline negative association between the adiponectin serum levels and uncontrolled asthma. Furthermore, adiponectin was significantly reduced in the obese women and in the group with the highest subcutaneous and visceral fat deposits. We also observed that adiponectin was negatively associated with BMI and subcutaneous fat after controlling for independent variables. In contrast, Holguin et al. did not find any association, independent of smoking and sex, between BMI and plasma adiponectin levels in 21 asthmatic patients.31 Nonetheless, Sideleva et al. did show a negative association, independent of BMI, of adiponectin gene expression in VAT of asthmatic obese level III patients compared with levels in an obese control group.24 We also observed higher levels of IL-6 in the group of patients with the highest percentile of visceral fat and a significant association between IL-6 and TAV after controlling for independent variables. Compared with abdominal SAT, VAT is considered to be more metabolically active and can produce various hormones and cytokines including TNF-α and IL-6.6,7 However, few studies to date have evaluated cytokines in asthmatics.5 Sideleva et al.24 also showed higher IL-6 expression in VAT compared with that in SAT in asthmatic patients. In another study, a positive association was found between IL-6 and TNF-α levels, and a negative association between adiponectin and metabolic syndrome was identified in women, which is similar to other studies.32–34
IL-6 production by VAT is associated with insulin resistance and metabolic syndrome, exhibiting additional associations with other inflammatory cytokines, including decreased adiponectin levels. Within this context, we believe that the VAT produced IL-6 may influence the pathophysiology of asthma in females and thereby make it more difficult to control asthma. Therefore, we hypothesize that obesity in asthmatic patients might be influenced by specific inflammatory cytokines associated with adipose abdominal tissue deposits in a manner similar to the chronic inflammation of adipose tissue that is observed in metabolic syndrome. We decided to include only females in this study. In contrast to men, females are influenced by hormonal changes throughout their entire lives,35 and it is possible that these changes result in a greater risk of developing non-atopic asthma and a worsening of symptoms as well as decreased lung function, and dysregulation of the immune response.36,37 In addition, by excluding patients with level III obesity, we aimed to reduce overestimation of asthma symptoms, respiratory function tests and comorbidities such as GERD and OSA, which are directly associated with obesity and asthma.12,38,39 One of the limitations of this study is its cross-sectional design, which does not allow determination of any causal relationship. It is also possible that the study included more severely affected and less well-controlled patients than is typical in general hospitals.
ConclusionIn conclusion, our results show a relationship between visceral obesity, poorly controlled asthma and decreased lung function in females. We also showed that IL-6 is associated with VAT and that adiponectin with SAT in asthmatic women. However, we cannot extrapolate if decreased pulmonary parameters and citokines are influenced by visceral obesity in asthmatic subjects, since we didn’t compare with nonasthmatic obese women. In addition, neither anthropometric measures nor subcutaneous adipose abdominal tissue was found to be associated with asthma control. We hypothesize that women should be studied as a separate group because they are influenced by continuous hormonal variations and are affected by a more severe inflammatory form of asthma. We suggest studies on obesity and asthma be performed using subjects from different geographical regions and various age groups. We also think that it will be important to assess the adipose tissue in different compartments with a more direct or gold standard measure of adiposity, such as magnetic resonance imaging. Finally, we recommend further studies with a control group of nonasthmatic obese women to determine if visceral adipose tissue is negatively associated with asthma and increased inflammatory markers.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Authors’ contributionsAV Capelo was responsible for the conception and design, acquisition of data. VM da Fonseca was responsible for the analysis and interpretation of data. MVM Peixoto was responsible for advising it critically for important intellectual content. SR de Carvalho was responsible for the final approval of the version to be published. CM Azevedo was responsible for the ultrasonographic abdominal measurements. MIG Elsas was responsible for the measurement of cytokine levels.