The gold-standard method for the diagnosis of exercise-induced bronchospasm (EIB) is an exercise test combined with spirometry. However, this test is expensive, time consuming and requires specialized equipment and trained personnel. Exhaled nitric oxide (eNO) is a fast, easy, noninvasive method for the diagnosis of EIB. The aim of the present study was to assess the accuracy of the measurement of eNO for the diagnosis of EIB through a systematic review of the literature.
MethodsA search was carried out in the PubMed, Lilacs, SciELO and SCOPUS databases by two independent researchers.
ResultsFifty-six papers were found. Following the application of the eligibility criteria to the title, abstract and text, six papers remained for analysis. There was a significant heterogeneity in sex (X2=56.44, p=0.000) and clinical spectrum (X2=504.00, p=0.000) between studies. In children between 3.8 and 7.8 years old a cutoff point >28ppb EIB can be ruled in and in children between 5 and 16 years old at a cutoff point <20EIB can be ruled out. For adults a cutoff point <7EIB can be ruled out and it can be ruled in with a cutoff point >12. Four papers reported negative predictive values above 88%.
ConclusionThe measurement of eNO seems to be effective for ruling in and ruling out EIB in some specific groups. Therefore, the meansurement of eNO levels could be an important tool to safely avoid the need for an exercise test when the result is negative, reducing the individual and economic impact of this disease.
Introdução: O método padrão de ouro para o diagnóstico de broncoespasmos induzidos por exercício (BIE) é a prova de esforço combinada com a espirometria. Contudo, esta prova é dispendiosa, demorada e requer equipamento específico e pessoal especializado. O óxido nítrico exalado (eNO) é um método rápido, simples e não invasivo para o diagnóstico de BIE. O objectivo do presente estudo foi o de aferir a acurácia do eNO para o diagnóstico do BIE através da revisão sistemática da literatura. Métodos: Foi efectuada uma pesquisa nas bases de dados PubMed, Lilacs, SciELO e SCOPUS por dois investigadores independentes. Resultados: Foram encontrados 56 artigos e após as exclusões pelo título, resumo e texto, restaram 6 artigos para análise. Foi encontrada heterogeneidade significativa entre os estudos quanto ao sexo (X2=56,44; p=0,000) e ao espectro clínico (X2 =504,00; p=0,000). Em crianças com idades entre os 3,8 e os 7,8 anos o ponto de corte >28 ppb pode considerar o BIE presente, e em crianças com idades entre os 5 e os 16 anos o ponto de corte <20 BIE poderá excluir essa afecção. Para adultos o BIE poderá ser excluído quando o ponto de corte for <7 BIE e o ponto de corte >12 poderá considerar a presença da doença. Quatro artigos registaram valores preditores negativos acima dos 88%. Conclusão: A avaliação do eNO parece ser eficaz na inclusão ou na exclusão de BIE em alguns grupos específicos. Assim sendo, a avaliação dos níveis de eNO poderá ser uma ferramenta segura para evitar a necessidade da prova de esforço nos casos negativos, reduzindo a impacto individual e económico desta doença.
Exercise-induced bronchospasm (EIB) is a transitory obstruction of the airways which occurs immediately after vigorous exercise; the main symptoms are breathlessness, cough, wheezing and chest tightness.1–4 The prevalence of EIB ranges between 5% and 20% in the general population, to 49% among individuals with asthma, reaching as high as 90% among those with persistent asthma.1,5 One hypothesis for this condition is the hyperventilation that occurs during exercise that leads to the inhalation of a large amount of cold dry air, which contrasts with the warm air in the lungs, thereby causing bronchospasm.1 This probably leads to the activation of inflammatory mediators, the stimulation of cholinergic receptors and edema in the airways, which causes bronchoconstriction.1,6
According to the American Thoracic Society, EIB should be diagnosed in the laboratory using an exercise test with a treadmill or exercise bike, in a setting with the temperature between 20°C and 25°C and humidity below 50%.3 EIB is confirmed using spirometry, comparing resting values with values found following exercise. Bronchoconstriction occurs when there is a reduction of 10–15% in the forced expiratory volume in the first second (FEV1) between 1 and 30min after the test.3,5 However, although accurate, this test is complex, expensive, time consuming and requires specialized equipment and trained personnel.7,8 There are risks and discomfort involved and some individuals may require medication and/or oxygen.8
The measurement of exhaled nitric oxide (eNO) is described in the literature as an alternative to the exercise test for the diagnosis of EIB.8–11 This is a non-invasive, fast, easy method that can be performed on adults and children over five years of age.8,10 Studies report the use of eNO for the adjustment of asthma medications, such as inhaled corticosteroids, as well as for the diagnosis of asthma.10,12,13 The test is also safe, it is not associated with risks such as inducing severe bronchospasm.9
Considering the impact of exercise-induced asthma has on quality of life particularly in children,14 this systematic review of the literature was carried out to assess the accuracy of the measurement of exhaled nitric oxide for the diagnosis of this condition.
MethodsA systematic review was carried out of studies which have assessed the accuracy of eNO for the diagnosis of EIB, whatever the language of the study. The standard reference for diagnosis was the exercise test with spirometry.
The inclusion criteria were, primary studies which compared the diagnosis of exercised-induced asthma or bronchoconstriction using eNO with the exercise test, and which included the determination of sensitivity and specificity. Cross-sectional and longitudinal studies with participants of any age were included, provided the diagnostic tests were performed on the same day. Studies involving animals, subjects with associated co-morbidities (with the exception of rhinitis) or smokers were excluded.
Search strategyThe studies were retrieved from the following databases: PubMed (1991 to August 2010), Latin American and Caribbean Health Sciences Literature (Lilacs) (1991 to August 2010), Scientific Electronic Library Online (SciELO) and SCOPUS. A general search strategy flexible enough to adapt to the characteristics of each database was used to identify studies which included the terms, exercise-induced asthma, exhaled nitric oxide and diagnosis.
The following strategy was used in the PubMed and SCOPUS databases: (Exhaled nitric oxide OR nitric oxide) AND (Exercise-Induced Asthma OR Exercise-Induced bronchoconstriction OR Exercise-Induced bronchospasm) AND (diagnosis OR Sensitivity and Specificity OR Diagnostic Techniques and Procedures); strategy used in the Lilacs database: (“Broncoespasmo Induzido por Exercício” OR “Asma induzida por exercício”) AND (“/diagnóstico” OR “Sensibilidade e Especificidade”) AND “Óxido Nítrico”; strategy used in the SciELO database: ASMA INDUZIDA PELO EXERCICIO or ASMA INDUZIDA POR EXERCICIO or BRONCOESPASMO INDUZIDO PELO EXERCICIO [Assunto] and DIAGNOSTICO or SENSIBILIDADE or ESPECIFICIDADE or ACURACIA [Assunto] and OXIDO NITRICO or OXIDO NITRICO EXALADO [Assunto]. From the primary studies retrieved, the bibliographic references were searched to be able to include further papers.
Two researchers independently analyzed the titles, abstracts and complete texts of the papers retrieved. Divergences were resolved by consensus with a third researcher. Data collection was done by the researchers independently. The data were extracted and computed in a data extraction form for each study. The data extracted were: name of authors, publication year, country, inclusion criteria, age of patients, number of patients, study design, the characteristics of standard test and the measurement of eNO, the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) questions, the cutoff point of eNO levels, the number of true positives, true negatives, false positive and false negative patients. Review Manager program (RevMan, version 5.0.20) was used to analyze the data to obtain sensitivity and specificity values and respective 95% confidence intervals.
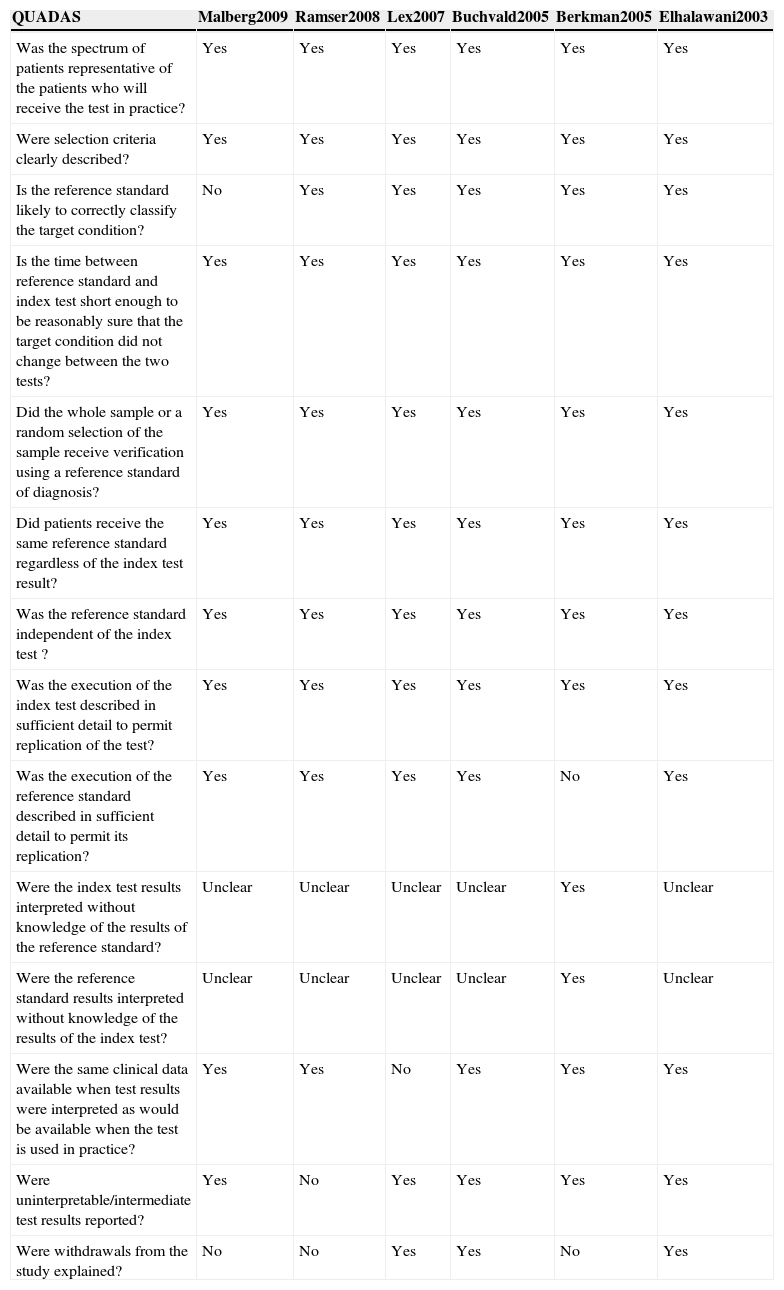
Quality assessmentThe quality of the papers was assessed using the QUADAS, which is a validated questionnaire made up of 14 items.15 The quality criteria were as follows: whether sample was representative of patient profile in clinical practice; clarity of the selection criteria for the participants; standard reference; time between evaluations with both methods; performance of all tests by the sample; independence of the tests; clarity of the protocols of the test studied and standard reference; blinding of the evaluators for both tests; information known to evaluators; non-interpretable results; and samples excluded. Two researchers were trained for the data extraction and use of QUADAS. Each item was identified as “yes”, “no” or “unclear”.16
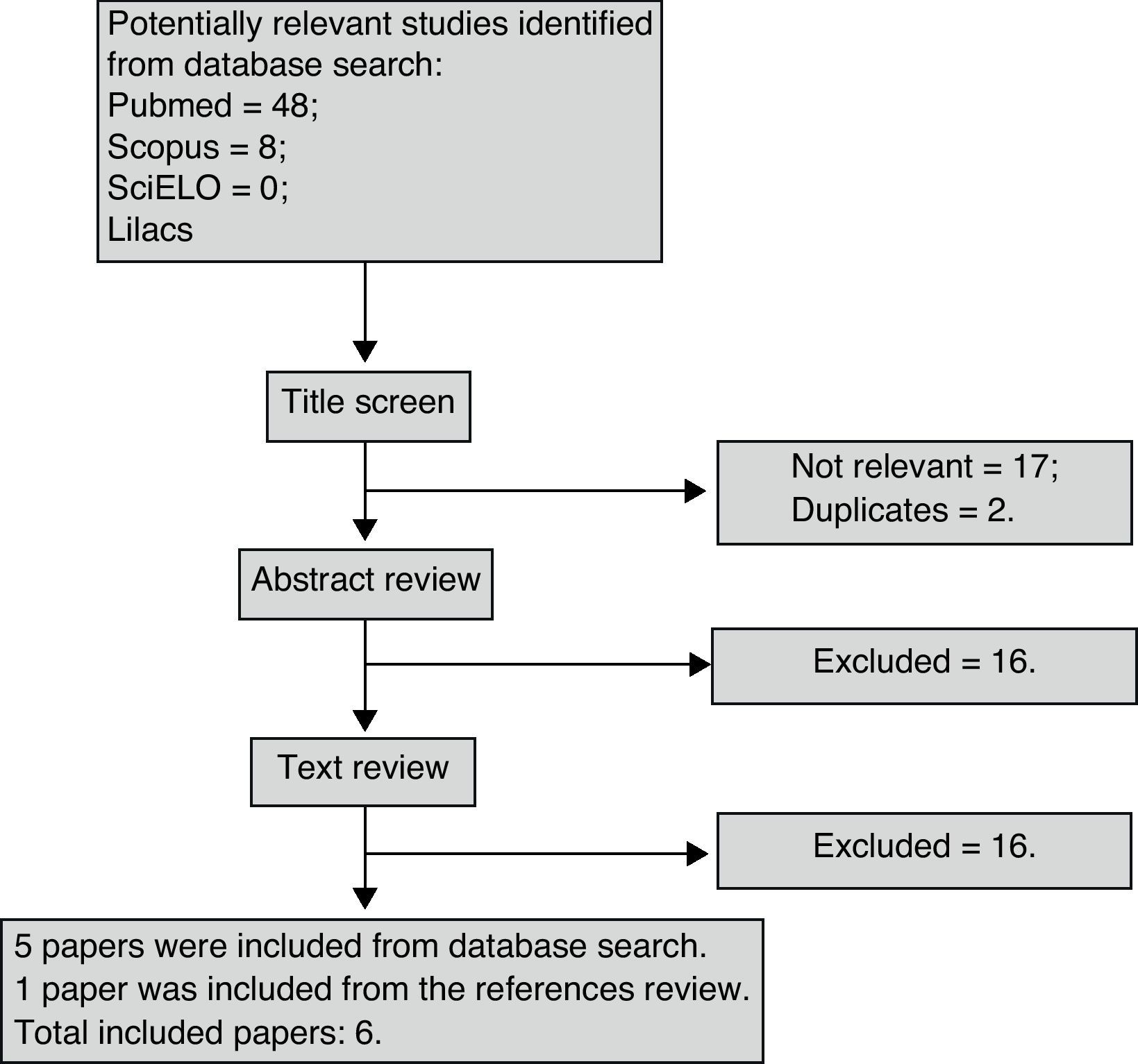
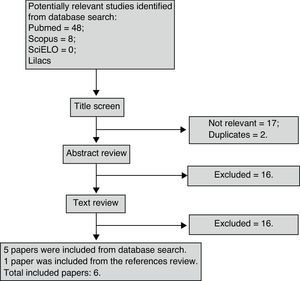
ResultsForty-eight studies were found in PubMed, eight in SCOPUS and none in either SciELO or Lilacs. One paper was found through the evaluation of the relevant references. Two papers were repeated which left 55 for analysis. Fig. 1 synthesizes the entire search process and paper selection.
Six papers met the eligibility criteria and 49 were excluded for the following reasons: 27 studies did not address accuracy; two did not compare eNO with exercise; seven were review articles; three did not use an acceptable standard reference; eight were not on EIB; and two were animal studies. The majority of studies that compared eNO with FEV1 values did not address diagnostic accuracy, as only two correlated the drop in FEV1 following exercise with baseline nitric oxide values. It was also impossible to construct a 2×2 table, as these studies did not present mean eNO values in the groups that were positive and negative for EIB.
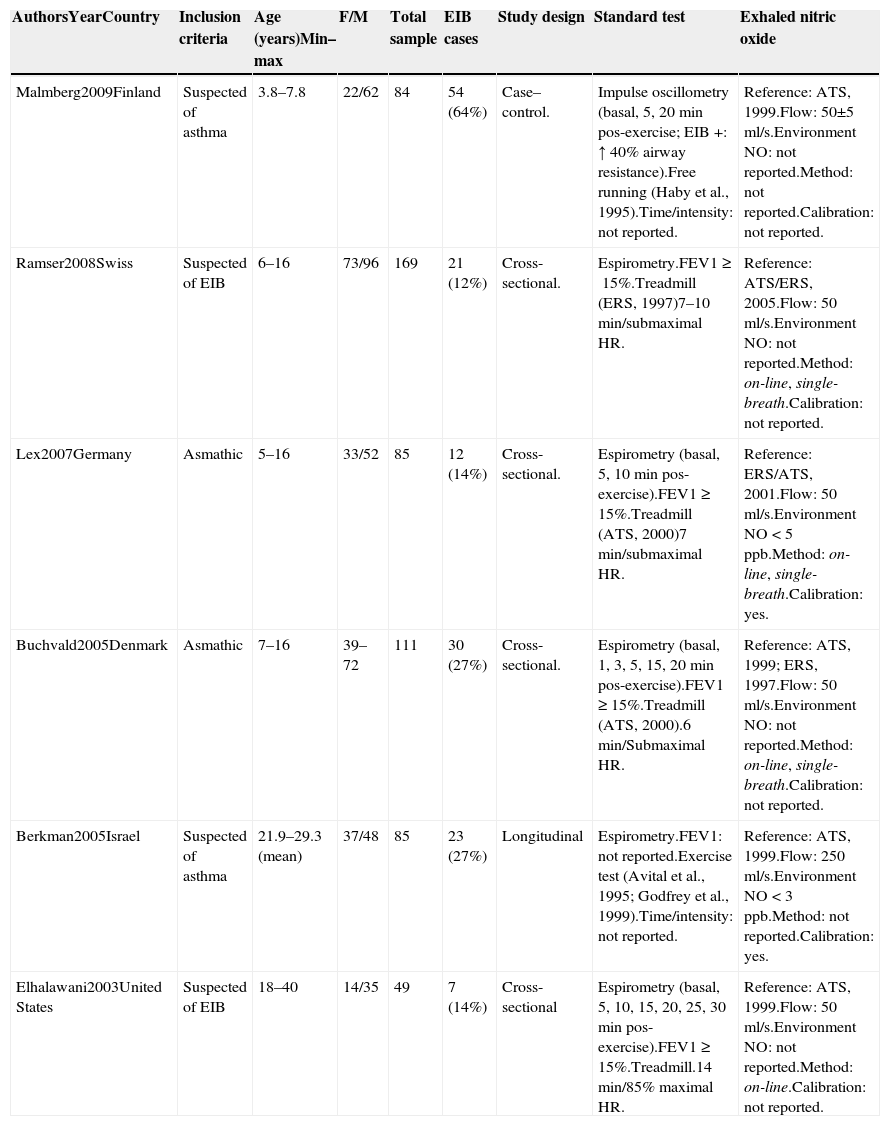
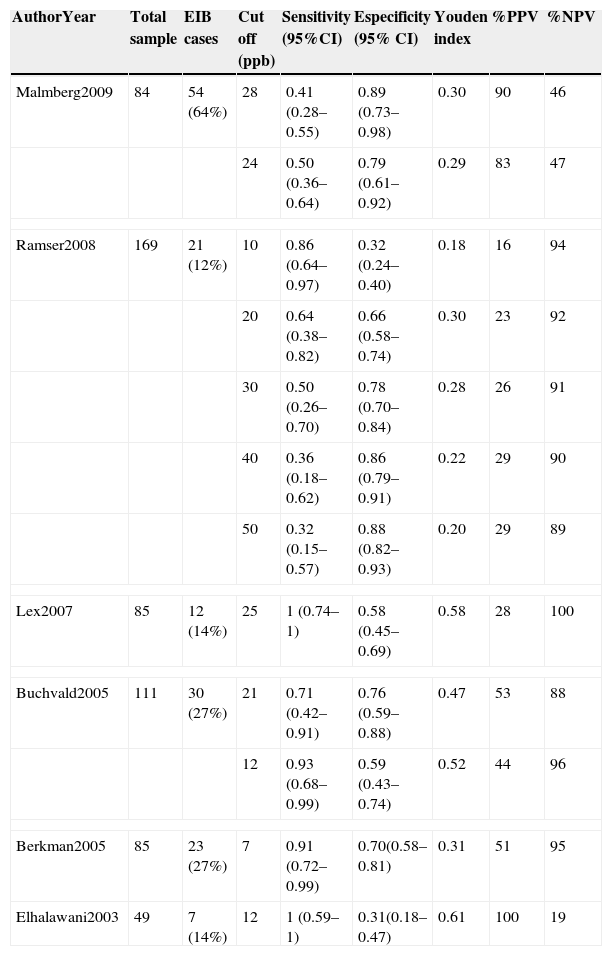
Six studies remained for analysis, involving a total of 672 individuals, 533 of whom were children and adolescents between 3.8 and 16 years of age. Table 1 displays the results of the quality assessment of the studies. Table 2 displays the characteristics of the studies. All studies were done in different countries. The age of patients ranged from 3.8 to 40 years old. There was a significant heterogeneity in sex (X2=56.44, p=0.000) and clinical spectrum (presence of asthma) (X2=504.00, p=0.000) between studies. The majority of the studies was cross-sectional and used a treadmill as the exercise challenge. Table 3 summarizes the accuracy results. The cutoff point of eNO levels ranged from 7 to 50 according to subgroups and age of patients. The sensitivity ranged from 0.32 to 1 between studies. The specificity ranged from 0.32 to 0.80. The highest predictive value (PPV) was 100% and the lowest PPV was 16%. The highest negative predictive value (NPV) was 100% and the lowest NPV was 19%.
Quality of studies.
| QUADAS | Malberg2009 | Ramser2008 | Lex2007 | Buchvald2005 | Berkman2005 | Elhalawani2003 |
|---|---|---|---|---|---|---|
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were selection criteria clearly described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the reference standard likely to correctly classify the target condition? | No | Yes | Yes | Yes | Yes | Yes |
| Is the time between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis? | Yes | Yes | Yes | Yes | Yes | Yes |
| Did patients receive the same reference standard regardless of the index test result? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the reference standard independent of the index test ? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the execution of the index test described in sufficient detail to permit replication of the test? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the execution of the reference standard described in sufficient detail to permit its replication? | Yes | Yes | Yes | Yes | No | Yes |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | Yes | No | Yes | Yes | Yes |
| Were uninterpretable/intermediate test results reported? | Yes | No | Yes | Yes | Yes | Yes |
| Were withdrawals from the study explained? | No | No | Yes | Yes | No | Yes |
QUADAS: Quality Assessment of Diagnostic Accuracy Studies.
Characteristics of included studies.
| AuthorsYearCountry | Inclusion criteria | Age (years)Min–max | F/M | Total sample | EIB cases | Study design | Standard test | Exhaled nitric oxide |
|---|---|---|---|---|---|---|---|---|
| Malmberg2009Finland | Suspected of asthma | 3.8–7.8 | 22/62 | 84 | 54 (64%) | Case–control. | Impulse oscillometry (basal, 5, 20min pos-exercise; EIB +: ↑ 40% airway resistance).Free running (Haby et al., 1995).Time/intensity: not reported. | Reference: ATS, 1999.Flow: 50±5ml/s.Environment NO: not reported.Method: not reported.Calibration: not reported. |
| Ramser2008Swiss | Suspected of EIB | 6–16 | 73/96 | 169 | 21 (12%) | Cross-sectional. | Espirometry.FEV1≥15%.Treadmill (ERS, 1997)7–10min/submaximal HR. | Reference: ATS/ERS, 2005.Flow: 50ml/s.Environment NO: not reported.Method: on-line, single-breath.Calibration: not reported. |
| Lex2007Germany | Asmathic | 5–16 | 33/52 | 85 | 12 (14%) | Cross-sectional. | Espirometry (basal, 5, 10min pos-exercise).FEV1≥15%.Treadmill (ATS, 2000)7min/submaximal HR. | Reference: ERS/ATS, 2001.Flow: 50ml/s.Environment NO<5ppb.Method: on-line, single-breath.Calibration: yes. |
| Buchvald2005Denmark | Asmathic | 7–16 | 39–72 | 111 | 30 (27%) | Cross-sectional. | Espirometry (basal, 1, 3, 5, 15, 20min pos-exercise).FEV1≥15%.Treadmill (ATS, 2000).6min/Submaximal HR. | Reference: ATS, 1999; ERS, 1997.Flow: 50ml/s.Environment NO: not reported.Method: on-line, single-breath.Calibration: not reported. |
| Berkman2005Israel | Suspected of asthma | 21.9–29.3 (mean) | 37/48 | 85 | 23 (27%) | Longitudinal | Espirometry.FEV1: not reported.Exercise test (Avital et al., 1995; Godfrey et al., 1999).Time/intensity: not reported. | Reference: ATS, 1999.Flow: 250ml/s.Environment NO<3ppb.Method: not reported.Calibration: yes. |
| Elhalawani2003United States | Suspected of EIB | 18–40 | 14/35 | 49 | 7 (14%) | Cross-sectional | Espirometry (basal, 5, 10, 15, 20, 25, 30min pos-exercise).FEV1≥15%.Treadmill.14min/85% maximal HR. | Reference: ATS, 1999.Flow: 50ml/s.Environment NO: not reported.Method: on-line.Calibration: not reported. |
Min–max: minimum–maximum; F/M: female/male; min: minutes; EIB: exercise induced bronchospasm; ATS: American Thorax Society; ml/s: milliliters/seconds; NO: nitric oxide; FEV1: forced expiratory flow in the first second; ERS: European Respiratory Society; HR: heart rate; ppb: parts per billion.
Results of included studies.
| AuthorYear | Total sample | EIB cases | Cut off (ppb) | Sensitivity (95%CI) | Especificity (95% CI) | Youden index | %PPV | %NPV |
|---|---|---|---|---|---|---|---|---|
| Malmberg2009 | 84 | 54 (64%) | 28 | 0.41 (0.28–0.55) | 0.89 (0.73–0.98) | 0.30 | 90 | 46 |
| 24 | 0.50 (0.36–0.64) | 0.79 (0.61–0.92) | 0.29 | 83 | 47 | |||
| Ramser2008 | 169 | 21 (12%) | 10 | 0.86 (0.64–0.97) | 0.32 (0.24–0.40) | 0.18 | 16 | 94 |
| 20 | 0.64 (0.38–0.82) | 0.66 (0.58–0.74) | 0.30 | 23 | 92 | |||
| 30 | 0.50 (0.26–0.70) | 0.78 (0.70–0.84) | 0.28 | 26 | 91 | |||
| 40 | 0.36 (0.18–0.62) | 0.86 (0.79–0.91) | 0.22 | 29 | 90 | |||
| 50 | 0.32 (0.15–0.57) | 0.88 (0.82–0.93) | 0.20 | 29 | 89 | |||
| Lex2007 | 85 | 12 (14%) | 25 | 1 (0.74–1) | 0.58 (0.45–0.69) | 0.58 | 28 | 100 |
| Buchvald2005 | 111 | 30 (27%) | 21 | 0.71 (0.42–0.91) | 0.76 (0.59–0.88) | 0.47 | 53 | 88 |
| 12 | 0.93 (0.68–0.99) | 0.59 (0.43–0.74) | 0.52 | 44 | 96 | |||
| Berkman2005 | 85 | 23 (27%) | 7 | 0.91 (0.72–0.99) | 0.70(0.58–0.81) | 0.31 | 51 | 95 |
| Elhalawani2003 | 49 | 7 (14%) | 12 | 1 (0.59–1) | 0.31(0.18–0.47) | 0.61 | 100 | 19 |
ppb: parts per billion; CI: confidence interval; PPV: preditive positive value; NPV: negative preditive value.
All papers included individuals who had similar characteristics to those who would do these tests in clinical practice and all clearly defined the inclusion criteria. Malberg et al.17 were the only ones not to use the ideal standard reference test; however, this study was not considered of low quality, because the participants were too young for the use of spirometry as the evaluation method. All studies had a sufficient time period between evaluations, used the same tests for comparisons and described the eNO protocol. Only Berkman et al.9 failed to describe the exercise test in detail; however, this was the only study to state clearly that the evaluators were blinded to both the eNO and standard reference results. In the majority of studies, the evaluators had information compatible with clinical practice and there were no non-interpretable results. Only half of the studies gave a detailed explanation of the reasons for excluded samples.
DiscussionThe measurement of eNO is an accurate negative predictor in the diagnosis of EIB in individuals with asthma or suspected EIB. As this test is easy to administer, is cheaper than the exercise test and can be administered to both children and adults without risk, it can be used as a screening tool for EIB. Thus, individuals with suspected EIB should have their eNO measured; if the value obtained is low, this condition can be ruled out. For patients with high eNO values, an exercise test should be performed to confirm the diagnosis of EIB.
All papers included in this systematic review followed the recommendations of the American Thoracic Society from 1999 to 2005 regarding the protocol for the measurement of eNO.18,19 The majority of studies used a flow rate of 50ml/s and the online single-breath method for the measurement of eNO. Only two papers specified the ambient NO value,8,9 which was lower than five parts per billion (ppb), as recommended by the American Thoracic Society and the European Respiratory Society.20 Among the six papers analyzed, only two reported the calibration of the eNO analyzer.8,9
As the clinical spectrum and sex of the groups was so heterogeneous, a meta-analysis of the studies was not considered appropriate. It was not possible to perform the heterogeneity evaluation for age and eNO cut off point because only one study described the mean and standard deviation of age and none of the studies provided these variables for eNO. It is possible that identifying an ideal cutoff point for the population studied will lead to greater accuracy in the use of eNO for the diagnosis of EIB. Thus, further studies should investigate the ideal cutoff point for adults and children with asthma, for those with allergies and those using inhaled corticosteroids.
The best way to diagnose intolerance to exercise is for the subject to take exercise, ideally in the context in which the brochospasm has been observed.21 However, this type of diagnosis is difficult to perform in clinical practice and other diagnostic methods that reduce the evaluation time; risk and cost have obvious advantages.
Among the six papers analyzed, four involved children and adolescents. Higher cutoff points were used for children and adolescents (21ppb) compared to adults. The same was true for individuals with asthma and allergies. Lower cutoff points should be employed for individuals who use inhaled corticosteroids, for, according to Silkoff et al.,22 the use of this medication for the treatment of asthma reduces eNO, as shown by the comparison of values before and after treatment. Another point to consider regarding the variability in the results is the issue of different temperature and humidity values, which may affect the prevalence of EIB and alter the predictive values of the tests in each study.23,24 All the studies used were carried out in different countries and there were no information about the time of year in which the evaluations were carried out.
Five of the studies report sensitivity values ranging from 0.86 to 1.0.7–11 However, these values were obtained at the cost of low specificity (0.31–0.7). Moreover, the studies that report high specificity values found low sensitivity values.7,17 Thus, the Youden Index was calculated to identify the studies with the best sensitivity and specificity relationship and only three studies achieved values above 0.5. These studies evaluated children using cutoff points of 1210,11 and 258ppb.
Four of the six papers report a NPV above 88%, reaching as high as 100% in the study carried out by Lex et al.8 Only the study by Elhalawani reports a low NPV, but reports a PPV of 100%. None of the other papers report a high PPV, which varied considerably among the different studies. For children between 3.8 and 7.8 years old, a cutoff point >28ppb could be considered a ‘rule-in’ for EIB, with the highest PPV of 90% and specificity of 0.89. In children between 5 and 16 years old at a cutoff point <20 EIB can be ruled out with a NPV between 94 and 100%. For adults between 21 and 40 years old, a cutoff point <7 EIB can be ruled out with a NPV of 95% but a cutoff point >12 would be a ‘rule in’ for a PPV of 100%.
Quality of the systematic reviewThe search for articles was sufficiently broad and carried out in four different databases. Emails were sent to all the authors whose studies were included to request unpublished papers. No restrictions were placed on the language or year of the studies. Only studies published since 1991 were selected, as this was the year in which the first paper on eNO in humans was published.25 All papers selected were evaluated by two researchers in an independent fashion. Meta-analysis was not possible, as the studies were carried out with different cutoff points. The authors were contacted by email for a new analysis of the cutoff point, but there was no response. The present review systematically followed the QUADAS16 criteria. All these factors contributed to the quality of the systematic review and validity of the study.
Implications for clinical practice and researcheNO is a simple, noninvasive method that can help reduce the number of people having to do an exercise test combined with spirometry. Individuals with a negative diagnosis determined by the measurement of eNO can be considered as not having EIB, whereas those with a positive result should be sent to do the exercise test to confirm the diagnosis. In clinical practice, this method can be used for both the diagnosis of this condition and the administration of medication when necessary, especially among individuals with asthma. The ideal cutoff point for the diagnosis of EIB depends on the population studied, as eNO varies among healthy individuals, those using corticosteroids, those with asthma and those with allergies and also varies between children and adults. Thus, further studies with large samples are needed to standardize the ideal cutoff points of eNO in different populations.
ConclusionThe measurement of eNO seems to be an effective test to ‘rule-in’ or rule out EIB in some specific groups. EIB is a strong possibility where there is a cutoff point >28 for children under 8 years old and a cutoff point >12 for adults. EIB could be ruled out with a cutoff point <20 for children between 5 and 16 years old and with a cutoff point <7 for adults. To standardize the ideal cutoff points of eNO in different subgroups of patients more studies with large samples are needed. The measurement of eNO for the diagnosis of EIB could be considered mainly an accurate negative predictor of this condition, but it is not possible to confirm the diagnosis with certainty in positive cases without an exercise test combined with spirometry. Therefore, the measurement of eNO levels could be an important tool for safely avoiding the exercise test when the result is negative, reducing costs and the personal impact of this disease.
Conflict of interestThe authors have no conflicts of interest to declare.