Chronic obstructive pulmonary disease (COPD) is currently a complex, multicomponent disorder. The COPD Assessment Test (CAT) has been increasingly used to assess COPD patients. This study aims to investigate the relationship between CAT and inflammation markers and other COPD components.
MethodsWe enrolled 110 stable COPD patients and 65 control subjects in this study. All patients completed the CAT questionnaire and the modified Medical Research Council (mMRC) dispnea scale. The quality of life of these patients was measured with St. George's Respiratory Questionnaire (SGRQ). Levels of TNFα, IL-6, CRP were determined in blood samples.
ResultsIn COPD patients, serum levels of TNFα (109.5 ± 58 pg/ml), IL-6 (10.3 ± 18 pg/ml), and C-reactive protein (CRP) (1.6 ± 1.7 mg/L) were found to be significantly higher compared to controls (TNF-α: 14.6 ± 18 pg/ml, IL-6: 2.14 ± 1.9 pg/ml, CRP: 0.4 ± 0.3 mg/L, p < 0.001). These markers were correlated with smoking (r from 0.27 to 0.35, p < 0.001), FEV1 (r from −0.39 to −0.57, p < 0.001), FVC (r from −0.32 to −0.37, p < 0.001) and FEV1/FVC (r from −0.31 to −0.66, p < 0.001). The CAT score correlated with GOLD spirometric stages, mMRC dyspnea score, number of exacerbations in the previous year and FEV1 (p < 0.001). There was a significant correlation between levels of CRP and the CAT score (r = 0.43, p < 0.001) but no similar relationship between levels of TNFα and IL-6 and the CAT was observed.
ConclusionSystemic inflammation persists in the stable period of COPD. CRP, one of the inflammation markers, was correlated with the CAT. Further studies are required to confirm the relationship between CAT and biomarkers.
Chronic obstructive pulmonary disease (COPD) is a significant cause of mortality and morbidity across the modern world. The mortality rate of this disease is increasing and it is predicted that it will become the third leading cause of death worldwide by 2020.1 It is not possible to define COPD based solely on forced expiratory volume in the 1s (FEV1) so the Global Initiative for Chronic Obstructive lung Disease (GOLD) has devised a multidimensional definition to assess COPD.2 The new definition includes the prevalence of symptoms and the previous history of exacerbations in addition to the FEV1 value and also emphasizes the assessment of comorbidities. In order to assess for symptoms, either the modified Medical Research Council (mMRC) or the COPD Assessment Test (CAT) is recommended. Additionally, a disease-specific quality of life scale (SGRQ, SF-36, CCQ) was used to evaluate the impact of the disease on daily life. Some studies have demonstrated a strong relationship between CAT and St. George's Respiratory Questionnaire (SGRQ).3, 4 Ladeira et al.5 showed that CAT was correlated to the BODE index.
COPD is a complex disease and includes genetic, cellular and molecular components. There are many different cells and molecules involved in the inflammatory pathways. Several indicators have been used to demonstrate a potential disease-related systemic inflammation.6, 7, 8, 9 Airway and lung parenchymal inflammation is a major pathogenic mechanism of COPD. In addition, persistent systemic inflammation may be associated with a various extrapulmonary comorbidities and pulmonary effects.10 Recent research has provided proof of the existence of distinct “novel systemic inflammatory COPD phenotype.” 7 In a recent study, it has been shown that cardiovascular comorbidity and diabetes were associated with higher levels for some markers of systemic inflammation.11 Therefore, genetic load, systemic inflammation and comorbid diseases are associated with various phenotypes in COPD. It is essential that new perspectives should be developed in the management of this disease.
Currently, CAT has been used increasingly for assessing and monitoring COPD. Although several serum biomarkers have been defined in COPD, there is not one single sufficient and effective single biomarker that can be used to assess the status of COPD patients. It has been shown that biomarkers are associated with several parameters such as inflammation, hospitalization, and mortality.7, 8, 9, 10, 11, 12, 13 However, the relationship between CAT and biomarkers has not been clearly demonstrated. Therefore, the present study aims to investigate the levels of systemic inflammation in COPD and determine the relationship between CAT and inflammation markers and quality of life.
Materials and methodsStudy designThis was a cross-sectional single-visit observational study. One hundred and ten patients with COPD diagnosed according to GOLD criteria14 and sixty-five control subjects were recruited between February 2013 and August 2013. Control subjects were chosen from those referred to a pulmonology and internal medicine clinic at Balikesir University Hospital, undergoing routine investigations. Written informed consent was obtained from all participants and the study was approved by the Institutional Ethics Committee of the Faculty of Medicine at Balikesir University.
All patients were subjected to physical examination, chest X-ray, respiratory function test, and routine blood analysis tests. The number of exacerbations in the previous year and the history of smoking were recorded. The CAT, mMRC dyspnea score was carried out via face-to-face interviews by pulmonary specialist. SGRQ scores were reported by the patients. For the healthy control group only a respiratory function test and routine blood analysis tests were done.
The population of the studyInclusion criteria: Patients were included if they; (1) were older than 40 years; (2) were current or ex-smokers with a smoking history ≥10 pack-years; (3) exhibited a post-bronchodilator FEV1 < 80% and an FEV1/FVC < 0.7. Control subjects were included if they: (1) were older than 40 years; (2) were free from lung disease as determined by a physician; (3) had a normal spirometry (FEV1 > 85% and FEV1/FVC > 0.7); and (4) had a smoking history of <5 pack-year.
Exclusion criteria: Patients were excluded who; (1) had an exacerbation of COPD within the previous 6 weeks; (2) had a respiratory disorder other than COPD or malignancy; (3) had a chronic inflammatory disease (vasculitis, inflammatory bowel disease, rheumatoid arthritis etc.) (4) had uncontrolled or severe concomitant disease (MI, arrhythmia etc.).
MeasurementsDemographic features, age, gender, smoking habits, admission to an emergency service or hospitalization over the past year, accompanying diseases, and existing treatments were all recorded appropriately. We defined an exacerbation as worsening of symptoms that required oral corticosteroids and/or antibiotics and/or hospitalization. Comorbid diseases were established using the clinical history and physical examination findings during the visit and were supported by a review of the available medical records. Current medications including inhalers, antihypertensive or other medications were documented. The height, weight, and BMI indexes were measured, and a spirometry was also performed in accordance with the international guidelines (ATS/ERS).15 The disease was classified according to the old and new version of GOLD staging.14 First, GOLD spirometric staging (FEV1 based (1–4) staging) and second, GOLD staging (A–D class). Dyspnea was assessed by the mMRC dyspnea scale.16 The patients with COPD were categorized into A, B, C and D combining symptom assessment by mMRC dyspnea scores and exacerbation risk.
CAT: The validity and reliability of the Turkish version of this scale has been previously verified.17 The CAT includes 8 items and yields total scores ranging from 0 to 40; higher scores indicate a more severe health status impairment or less successful control of COPD.3
Quality of life Questionnaire: The quality of life was assessed by using the Turkish translation of St. George's Respiratory Diseases Questionnaire (SGRQ).18
BiomarkersWe chose the biomarkers based on previous studies.7, 9, 10, 11, 12, 13 The blood samples taken from all subjects were centrifuged and stored at −80 °C. All samples were analyzed when the study was completed. The serum was studied in a diagnostic device (BioTek, ELx 800, USA) with use of commercial kits (eBioscience, Human TNF-α and Human IL-6 Platinum ELISA, Austria), and with the methods of tumor necrosis alpha (TNF-α) and an interleukin-6 (IL-6) level enzyme linked immunosorbent assay (ELISA). The levels of C-reactive protein (CRP) were evaluated with a clinical chemistry analyzer (Cobes Integra 800, Roche diagnostics) using a commercial kit. The reference value of CRP is 0–0.5 mg/L, while the analyzed lowest value of IL-6 is 0.92 pg/ml. The analyzed lowest value of TNFα is 2.3 pg/ml. TNF-α and IL-6 concentrations of some samples were below the lower limit of quantification (LLQ). In the analysis of individuals with values below the LLQ, a nominal level of half of the LLQ value was used to avoid a downward bias of the population data.19
Statistical analysisThe average CRP values of COPD and control groups (3.2 (1.5,7.1), 1.3 (0.6,2.7)) were identified from similar studies.7 After that, in each group, the sample size was calculated by using average calculating formula with 80% accuracy and 5% error. Accordingly, the sample size was found to be at least 55 subjects in each group. Results are presented as mean ± SD, median, or percentage, as appropriate. The Student's t-test and ANOVA were used for parametric tests; the Mann–Whitney U-test, Kruskal–Wallis and chi-square statistics were used for non-parametric tests for group comparisons. Fisher's exact test evaluated the differences between the percentages of comorbidities and the differences between sexes. The chi-square test evaluated the differences between the percentages of comorbidities. Pair-wise correlation of continuous variables in patients with COPD was examined by Pearson correlation. A value of p < 0.05 was considered statistically significant. All statistical analyses were performed with the SPSS (version 20.0) software.
ResultsClinical characterization of subjectsThe study included 110 patients (mean age of 64 ± 8.9 years; 100 male (90.9%)), and 65 control subjects (mean age of 61.5 ± 9.2 years, 55 male (84.6%)). The demographic and clinical characteristics of the patient and control groups are given in Table 1. Forty-five (40.9%) patients with COPD had an accompanying disease (Ischemic heart disease (n = 21), hypertension (n = 13), diabetes mellitus (n = 8), other (n = 3)). The mean FEV1 was 48.8% of the predicted value and mean FEV1/FVC: 55.4% in the COPD group. The mean CAT score was 22.6 (±9.2), mMRC: 1.9 (±0.9) and total SGRQ: 58.4 (±22.2). Of the 110 patients, 9.1% were stage 1, 37.3% were stage 2, 42.7% were stage 3, 10.9% were stage 4 according to GOLD spirometric staging. When patients were classified with respect to GOLD staging, 27.3% were group A, 30.9% were group B, 7.3% were group C and 34.5% were group D.
Table 1. Demographic, functional, clinical features of the patient and control groups.
| Patient (n = 110) | Control (n = 65) | p-value | |
| Age | 64.0 ± 8.9 | 61.5 ± 9.2 | 0.080 |
| Male n, (%) | 100 (90.9) | 55 (84.6) | 0.224 |
| BMI, kg/m2 | 26.5 ± 5.6 | 25 ± 2.6 | 0.064 |
| Pack-years | 38.9 ± 23 | 0.1 ± 1.2 | <0.001 |
| Co-morbidities, n (%) | |||
| Any | 65 (59.1) | 42 (64.6) | 0.95 |
| Ischemic heart disease | 21(19.1) | 11 (16.9) | |
| HT | 13 (11.8) | 6 (9.2) | |
| DM | 8 (7.3) | 4 (6.2) | |
| Other | 3 (2.7) | 2 (3.1) | |
| mMRC | 1.94 ± 0.9 | 0.04 ± 0.2 | <0.001 |
| FEV1, % predicted | 48.8 ± 17.8 | 89.4 ± 5.6 | <0.001 |
| FVC, % predicted | 68.7 ± 17.8 | 92.1 ± 5.8 | <0.001 |
| FEV1/FVC, % | 55.4 ± 12.9 | 89.6 ± 4.7 | <0.001 |
| GOLD spirometric stage, n (%) | |||
| 1 | 10 (9.1) | ||
| 2 | 41 (37.3) | ||
| 3 | 47 (42.7) | ||
| 4 | 12 (10.9) | ||
| GOLD stage, n (%) | |||
| A | 30 (27.3) | ||
| B | 34 (30.9) | ||
| C | 8 (7.3) | ||
| D | 38 (34.5) | ||
| SGRQ-total score | 58.4 ± 22.2 | ||
| CAT score | 22.6 ± 9.2 | ||
| TNFα (pg/ml) | 109.5 ± 58 | 14.6 ± 18 | <0.001 |
| IL-6 (pg/ml) | 10.3 ± 18 | 2.14 ± 1.9 | <0.001 |
| CRP (mg/L) | 1.6 ± 1.7 | 0.4 ± 0.3 | <0.001 |
Abbreviations: BMI, body mass index, HT: hypertension, DM: diabetes, FEV1: forced expiratory volume in 1 second, FVC: forced vital capacity, CAT: COPD assessment test.
There was no significant difference between the ages (p = 0.080, Student's t-test) and sexes of the two groups (p = 0.453, fisher's exact test) (Table 1). The groups exhibited similar incidences of comorbidities (p = 0.095, χ2 test). As expected, COPD patients had significantly lower pulmonary function parameters (FEV1, FVC, FEV1/FVC) compared to controls (p < 0.001, Student's t-test). In general, COPD patients exhibited higher serum levels of CRP (1.6 ± 1.7 mg/L) and TNF-α (109.5 ± 58 pg/ml) and IL-6 (10.3 ± 18 pg/ml) than healthy controls (CRP: 0.4 ± 0.3 mg/L, p < 0.001; TNF-α: 14.6 ± 18 pg/ml, p < 0.001; IL-6: 2.14 ± 1.9 pg/ml, p < 0.001, Student's t-test) (Table 1).
Association of CAT questionnaires and patient characteristicsThere was a correlation between the CAT score and the GOLD spirometric stage (Pearson's r = 0.43; p < 0.001). The CAT score increased in parallel with disease severity. There was a statistically significant relationship between the CAT score and number of exacerbations in the previous year (Pearson's r = 0.35, p < 0.001), disease duration (Pearson's r = 0.29, p < 0.001), smoking (pack-year) (Pearson's r = 0.27, p = 0.014) and mMRC (Pearson's r = 0.59, p < 0.001). The CAT score was found to be significantly correlated with FEV1 (Pearson's r = −0.39, p < 0.001) and FVC (Pearson's r = −42, p = 0.003). At the same time, a strong correlation was observed between the CAT score and the SGRQ symptom, activity, impact and total scores (Pearson's r = 0.72; p < 0.001).
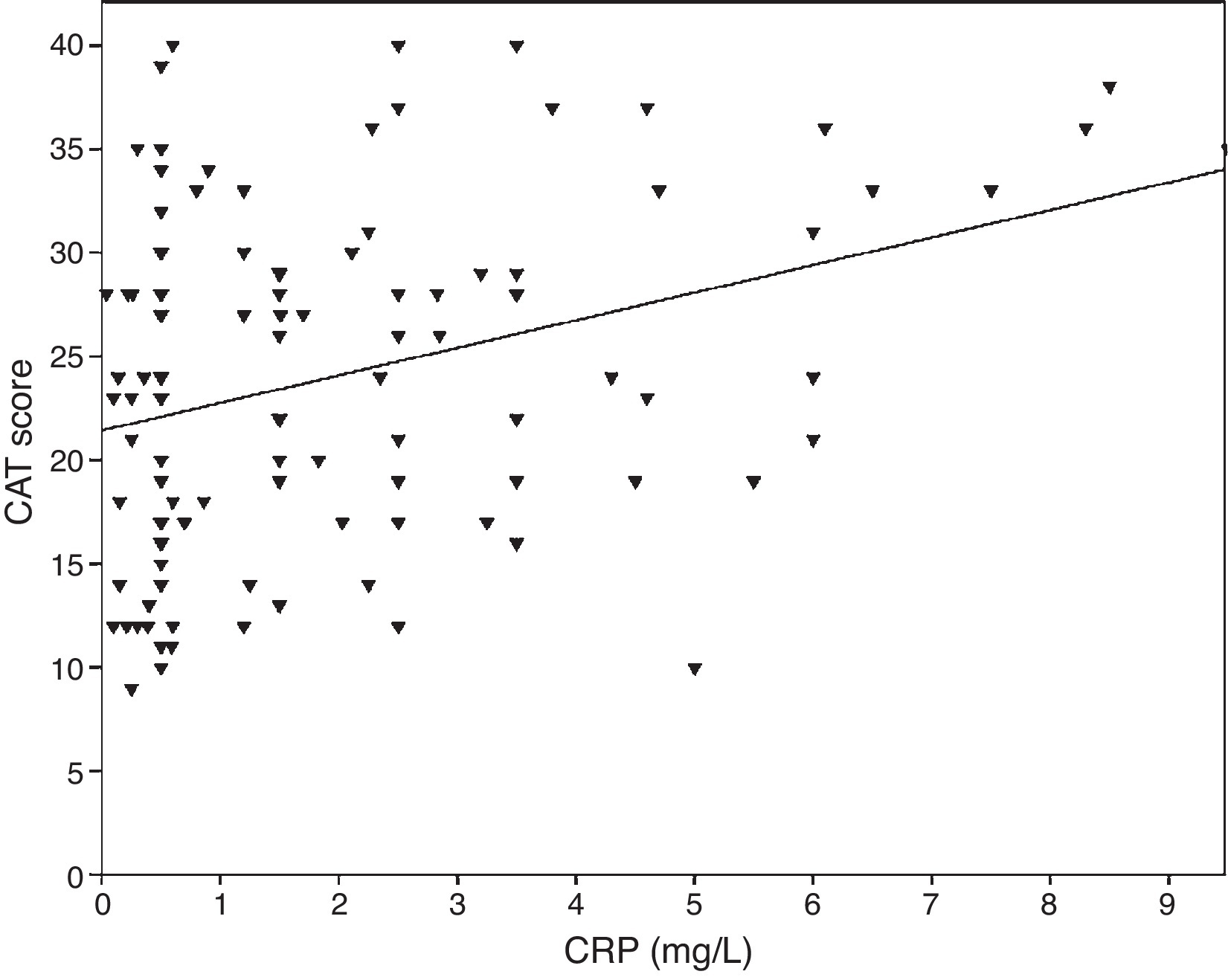
Association between biomarkers and patient characteristicsInflammatory markers and pulmonary function parameters were compared. As the FEV1 decreased, levels of biomarker increased significantly. CRP levels were correlated with FEV1 (Pearson's r = −0.39, p < 0.001), FVC (Pearson's r = −0.33, p < 0.001) and FEV1/FVC (Pearson's r = −0.38, p < 0.001). TNF-α levels were also correlated with FEV1 (Pearson's r = −0.57, p < 0.001), FVC (Pearson's r = −0.37, p < 0.001) and FEV1/FVC (Pearson's r = −0.66, p < 0.001). IL-6 levels were also correlated with FEV1 (Pearson's r = −0.31, p < 0.001), FVC (Pearson's r = −0.32, p < 0.001) and FEV1/FVC (Pearson's r = −0.34, p < 0.001). A significant correlation was observed between CRP, TNF-α, IL-6 and smoking (pack-year) (Pearson's r = 0.35, r = 0.44, r = 0.27, p < 0.001, respectively). COPD patients with cardiovascular disease had an increased level of CRP (2.98 ± 2.4 mg/L) compared to patients without comorbidities (1.56 ± 1.5 mg/L, p = 0.008 ANOVA test followed by LSD post hoc test). There was no significant association with other biomarkers and comorbidities. As the IL-6 increased, the mMRC score increased, but this relationship was not statistically significant (p = 0.06). When inflammatory markers were compared with CAT score, CRP levels were shown to have a significant correlation (r = 0.43, p < 0.001) (Figure 1) while no correlation was observed with TNF-α and IL-6. There was a correlation between CRP levels and TNFα (r = 0.48, p < 0.001). No direct association was observed between biomarkers and A-D class or spirometric stages.
Figure 1. The relationship between CAT scores and CRP (r = 0.43, p < 0.001).
DiscussionSome assessment tools are used such as clinical tests (CAT, BODE), inflammation markers and quality of life questionnaires to evaluate COPD. It is commonly accepted that a multidimensional assessment is required to understand and manage the disease. COPD is a complex disorder with a systemic component and some patients appear to have an inflammatory phenotype. In this study, CAT was performed as a new clinical test, and the relationship of CAT with other COPD components was investigated.
One of these components is systemic inflammation. The biomarkers most often studied in COPD to indicate systemic inflammation which are commonly used are CRP, IL-6, TNF-α, IL-8 and fibrinogen. Several studies have previously reported elevated circulating levels of these markers in patients with stable COPD.6, 7, 8, 9, 20, 21, 22, 23 In a recent study, Agustí et al. showed that 30% of COPD patients did not present evidence of systemic inflammation and 16% of the patients did present persistent systemic inflammation.7 Interestingly, in this study, the serum levels of TNF-α and IL-8 were found to be higher in smokers with normal spirometry compared to COPD patients. The other markers as white blood cells, IL-6, CRP and fibrinogen were found to be higher in COPD patients than in the smokers with normal spirometry and non-smokers. In our study, the levels of CRP, TNF-α, IL-6 were significantly higher in all COPD patients when compared to the control group. All the biomarkers were found to be correlated with the cumulative smoking exposure and reduction of pulmonary function test parameters (FEV1, FVC, and FEV1/FVC). The biomarkers level relate to the degree of airflow obstruction. These results are consistent with those reported in previous cross sectional studies.13 In another study which examined the results of ECLIPSE, cardiovascular comorbidities and diabetes were correlated with some systemic inflammation markers.9 In our study, COPD patients with cardiovascular disease had an increased level of CRP compared to patients without comorbidities. Increased systemic inflammation in COPD with cardiovascular diseases support a pathophysiological mechanism between COPD and these comorbidities.
The relationship between CAT and systemic inflammation has not been clarified by the studies conducted so far. In one study, a significant correlation was shown to exist between the LCN1, LCN2 and CAT.8 In our study, a significant relationship has been shown between CAT and CRP. However, a similar correlation could not be shown with TNF-α and IL-6.
In the present study, COPD patients were categorized into four groups (A–D) and stages (1–4) according to old and new version of GOLD classification. It can be observed that Stage 1 and Stage 4 constitute the smallest proportion of the population with 10% each according to the old classification. In the new version, the population was distributed almost equally among groups A, B and D; the smallest distribution was that of group C. In other studies, it has also been shown that group C constitutes the smallest proportion of the distribution.24, 25 The new classification indicates that there may be some patients with multiple symptoms but mild airway obstruction as well as some patients with few symptoms but with severe airway obstructions. Comorbidities and systemic inflammation can lead to increases in exacerbation and symptoms in patients with mild/moderate obstruction, and in this way, it causes these patients to be placed in the high risk groups (C or D class). In one study, it was shown that subtype C includes patients with higher comorbidity status and subtype D includes patients with the most severe exacerbation, a high rate of exacerbation related to hospitalization and poorest outcomes.26 In a recent study, Agustí et al. compared two groups with and without persistent systemic inflammation and showed that patients presenting persistent inflammation during follow-up had increased exacerbation rates per year compared to the other group although pulmonary abnormalities were similar in these two groups.7
GOLD recommends the use of CAT or mMRC scale to assess symptoms. We used mMRC scale for group assignment, because when we considered the symptoms on the basis of CAT, the number of the patients with less than 10 breakpoints was very small (8 patients). The higher CAT scores may be associated with the higher perceptions of patients’ symptoms. It was also shown that CAT and mMRC are not equivalent, and this may cause some differences in classification.24, 25, 27 In the new classification, the other cut-off points are composed of number of exacerbations per year. The relationship between basal CAT score and the frequency of exacerbations was shown in the COPD patients.28 Pothirat et al. have shown that the change in CAT score during monitoring visits is a useful tool for detecting acute deterioration in health status of COPD patients.29 In our study, a strong relationship was observed between CAT, mMRC and the exacerbation rates. The CAT was also found to be correlated to the duration of illness, and smoking (pack-year). The mean FEV1 of the patients was found to be 48.8% of the predicted value and the mean CAT score was 22.6. A strong association was shown between CAT and FEV1, FVC and the GOLD spirometric stage. As the FEV1 and FVC decreased, the CAT score increased. The CAT score also positively correlated with a heavier GOLD stage. These results show that CAT reflects the severity of disease very well.
Another important component of COPD management is quality of life. A variety of life-questionnaires have been used to evaluate the effects of the disease on daily life. The reliability and validity of the Turkish version of SGRQ has been proved.30 A few studies have shown that significantly correlation was observed between CAT and SGRQ.3, 4 In a study, it has been reported that CAT is sensitive to the change in health status associated with COPD exacerbations.31 In our study, a strong relationship was shown between CAT and SGRQ. Since CAT, compared to SGRQ, is a shorter and easier test to understand, the use of CAT is more practical.
The present study has several limitations, such as cross-sectional design, a single center study and small sample size. In addition, the inflammation markers could not be compared in smokers who have normal lung function because they were not included in the present study. The medications for patients may exert an influence on systemic inflammatory response and health status and they may also affect the results.
In summary, CAT is a test that can be used in the assessment of COPD, since its reliability and validity have been clearly demonstrated. The results of our study indicate the relationship between CAT and CRP as well as other COPD components (clinical, functional parameters). However, longitudinal multicenter studies are required to evaluate the relationship between CAT and biomarkers.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
AuthorshipStudy design: NS, FE and AAH; Data collection: NS, CB; Data analysis and interpretation: NS, AAH, and CB; Critical revision of the manuscript: NS, FE, CB and AAH.
Conflicts of interestThe authors declare that they have no conflict of interest in the preparation of this manuscript.
Acknowledgements
This study was supported by Balikesir University Research Funds (Project number: BAP 2012/95). The authors thank Prof. Dr. A. Said BODUR from Balikesir University, Faculty of Medicine, Department of Public Health for his assistance in statistical analysis.
Received 28 April 2015
Accepted 2 August 2015
Corresponding author. nurhangencer@hotmail.com