In 2012 Portugal had a tuberculosis incidence of 21.6/100,000 inhabitants (2286 new cases, 942 of which were reported in the northern region).1 A recent retrospective study in Portugal identified, from 2001 to 2012, 25 cases of tuberculosis out of 765 patients under anti-TNFα (297/100,000 patient-years).2
Treatment with tumor necrosis factor antagonists (anti-TNFα) is associated with an elevated risk for development of tuberculosis mostly due to reactivation of latent tuberculosis infection. Since 2006, national guidelines advise tuberculosis screening for all candidates for anti-TNFα therapy including chest X ray, tuberculin skin test (TST) and Interferon-Gamma Release Assay (IGRA) and treatment should be offered to every patient with evidence of LTBI, provided major toxicity is excluded.3
Worldwide, the application of tuberculosis screening guidelines in these patients has been related to a decrease of tuberculosis within this group.4,5
In order to understand the pitfalls that can still lead to the development of tuberculosis in these patients, we reviewed all cases of tuberculosis between 03/2010 and 02/2012 in Portugal's northern region, identifying those who developed the disease while on anti-TNF-α therapy. In this region there are both clinical notification of all tuberculosis cases and laboratory notification of confirmed cases (which allowed for the identification of all patients). Demographic, epidemiological and clinical data was obtained by consulting epidemiological research studies and clinical records.
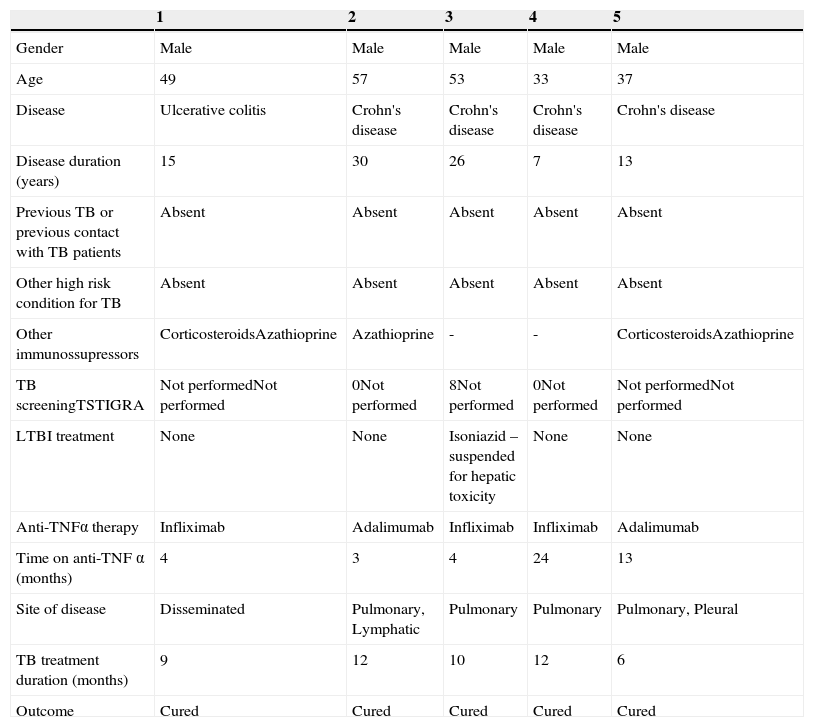
In the period studied, 1834 cases of tuberculosis were diagnosed in the region, five of which were receiving anti-TNF-α therapy (Table 1).
Patient data.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Gender | Male | Male | Male | Male | Male |
| Age | 49 | 57 | 53 | 33 | 37 |
| Disease | Ulcerative colitis | Crohn's disease | Crohn's disease | Crohn's disease | Crohn's disease |
| Disease duration (years) | 15 | 30 | 26 | 7 | 13 |
| Previous TB or previous contact with TB patients | Absent | Absent | Absent | Absent | Absent |
| Other high risk condition for TB | Absent | Absent | Absent | Absent | Absent |
| Other immunossupressors | CorticosteroidsAzathioprine | Azathioprine | - | - | CorticosteroidsAzathioprine |
| TB screeningTSTIGRA | Not performedNot performed | 0Not performed | 8Not performed | 0Not performed | Not performedNot performed |
| LTBI treatment | None | None | Isoniazid – suspended for hepatic toxicity | None | None |
| Anti-TNFα therapy | Infliximab | Adalimumab | Infliximab | Infliximab | Adalimumab |
| Time on anti-TNF α (months) | 4 | 3 | 4 | 24 | 13 |
| Site of disease | Disseminated | Pulmonary, Lymphatic | Pulmonary | Pulmonary | Pulmonary, Pleural |
| TB treatment duration (months) | 9 | 12 | 10 | 12 | 6 |
| Outcome | Cured | Cured | Cured | Cured | Cured |
All these 5 cases had intestinal disease (4 had Crohn's disease) of an average duration of 18.2 years (minimum 7 maximum 30). None of the patients had known exposure to tuberculosis, 3 patients underwent screening with TST but IGRA was never used. One patient was diagnosed with latent infection based on a TST of 8mm induration and started isoniazid but it was suspended 15 days later due to liver toxicity and anti-TNF-α was started. Patients developed tuberculosis between 3 and 24 months on therapy.
In none of the cases was screening performed according to the published guidelines, it was either absent or incomplete. Even though they represent a small fraction of TB diagnosis, the incidence in this group is significantly higher2 and a methodical screening and follow up while on biological therapy should be our goal. This is particularly important in a country like ours which has an intermediate incidence of TB.
The fact that all TB cases occurred in patients being treated for inflammatory bowel disorders may represent a lack of awareness of tuberculosis and of the guidelines by the clinicians treating these patients.