The hypervascularization of the bronquial wall, secondary to chronic bronchopulmonary inflammation is a bleeding etiology in smokers, but insufficient to explain certain massive recurrent cases. We report a case of a woman with a smoking history who presented a recurrent and massive hemoptysis. A diagnostic study with laboratory tests, bronchoscopy, computed tomography and echocardiogram did not identify the etiological cause. However, bronchial arteriography showed right and left bronchial tortuous and dilated arteries and demonstrated that a bronchovascular fistula was the origin of the hemoptysis. An acquired form of the Dieulafoy's disease in this context of a smoking history might justify such findings. Bronchial arteriography as a diagnostic method should be the preferred choice rather than bronchoscopy in these cases.
Hemoptysis is a frequent symptom in pneumology and requires rapid and careful intervention. There are numerous causes of bleeding from the lower respiratory tract. Bronchiectasis, tuberculosis, bronchogenic carcinoma, and various lung infections are still believed to be the most common causes of massive hemoptysis.1,2 A recent large series demonstrates the prevalence of the causes of massive hemoptysis in a university hospital setting.3 Further, depending upon the study, in as many as 30 percent of patients with hemoptysis no cause can be identified even after careful evaluation, including bronchoscopy. These patients are classified as having either cryptogenic or idiopathic hemoptysis.4 In cases in which no associated comorbidity can be confidently excluded, the subgroup of cryptogenic hemoptysis shares common risk factors with patients whose hemoptysis is explained by chronic bronchitis in a context of smoking history.5,6 Hypervascularization within the bronchial wall remains an ill-defined process in the pathogenesis of chronic airway inflammation, resembling the endobronchial findings described in Dieulafoy's disease.
We present a case of massive hemoptysis due to hypervascularization and a subsequent bronchovascular fistula, related to a smoking history.
Case reportWe report a remarkable case of 49-years-old women, housewife, with a smoking history (29 packs cigarettes/year), without other toxics exposure, presenting cough and abundant blood expectoration, which was not quantified, without other semiology.
In the emergency room, the patient was hemodynamically stable. She required supplemental oxygen therapy without noninvasive or invasive mechanical ventilatory support. Laboratory studies found a hemoglobin of 9.0g/dL, without thrombocytopenia or coagulopathy. The urgent bronchoscopy showed plentiful blood remnants on tracheobronchial tree and signs of chronic inflammation without active bleeding. Therefore, we decided to continue diagnostic evaluation because the bleeding had ceased and the patient remained clinically stable.
The patient had no telangiectasias on skin or mucous, and there was no family history of hemoptysis, brain aneurisms, epistaxis or gastrointestinal bleeding, which might have suggested possible hereditary hemorrhagic telangiectasia. Additional laboratory testing discarded other causes of hemoptysis, ranging from liver dysfunction, neoplasms or infection, to immune or inflammatory disorders. Radiological studies discarded regional hyperlucency or bullae. Moreover, computed tomography of the chest discarded those abnormalities that are difficult to detect with bronchoscopy, such as mass lesions (e.g., arteriovenous malformations, cancer or aspergilloma), bronchiectasis, lung abscess or pulmonary artery aneurysm. An echocardiogram discarded mitral stenosis, pulmonary hypertension, endocarditis or congenital heart disease. Pulmonary function testing showed a moderate (GOLD grade 2) chronic obstructive pulmonary disease (COPD).
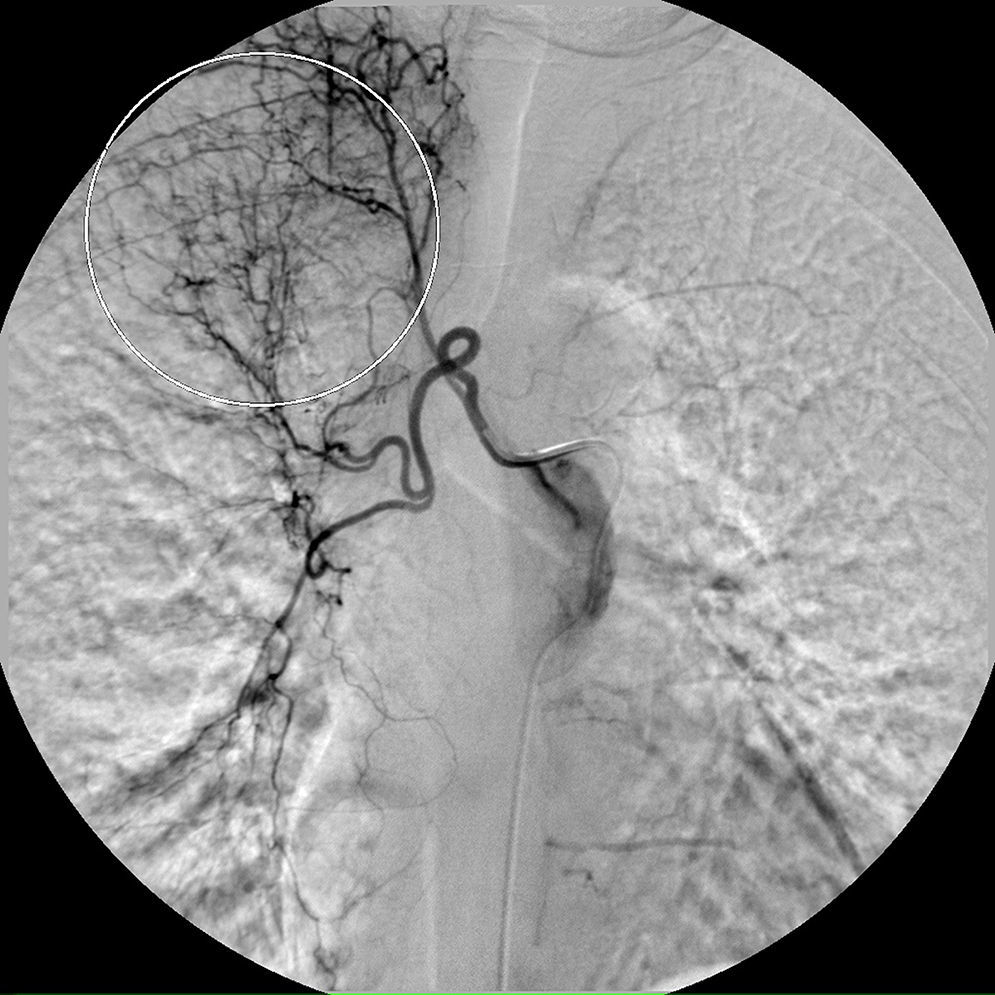
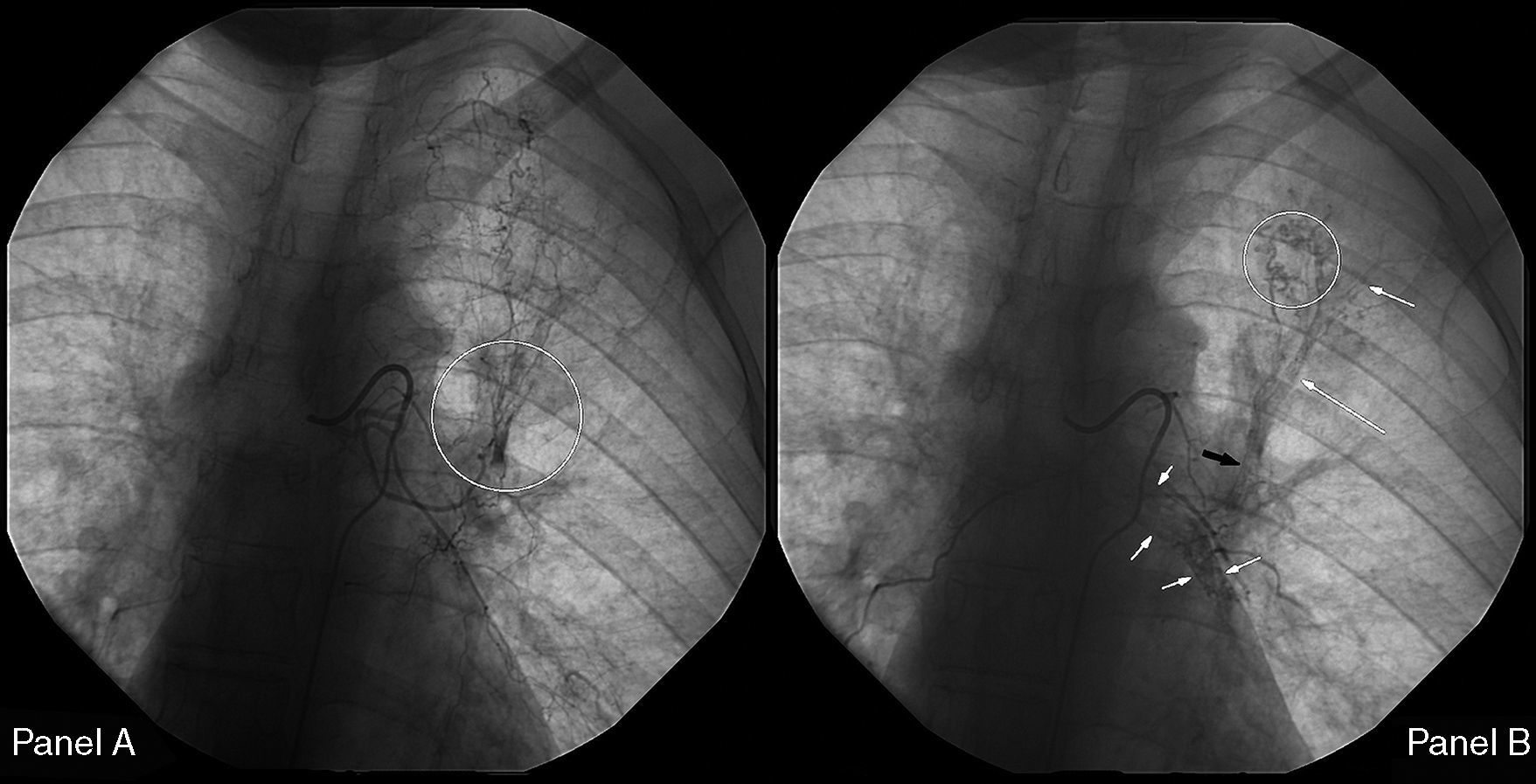
On the seventh day, a new episode of massive hemoptysis took place. On this occasion, the patient was hemodynamically unstable and the laboratory tests disclosed a hemoglobin of 7.3g/dL. Two units of “packed” red blood cells and sufficient volume of crystalloid fluid were administered. The patient refused another bronchoscopy. Urgent bronchial arteriography was performed. Selective catheterisation of the right intercostobronchial trunk showed features of marked hypervascularization, including a tortuous and dilated right bronchial artery and bronchial-to-pulmonary retrograde shunts in the right apex (Fig. 1). Selective catheterisation of the left bronchial artery showed a bronchovascular fistula, where contrast leaked from the blood vessel drawing the left bronchial tree bronchography (Fig. 2, panel A), and a tortuous and dilated superior division of left bronchial artery too (Fig. 2, panel B). Immediate cessation of hemoptysis was obtained after embolization of the bronchovascular fistula and the right intercostobronchial trunk with polyvinyl alcohol microspheres of 700–900μm until complete stasis of the artery. Following the procedure, the patient was stabilized hemodynamically and did not present new episodes of hemoptysis. After the last episode, the patient stopped smoking and she has remained asymptomatic until now.
Left bronchial arteriography. Panel A. Circle: bronchovascular fistula. Panel B. Black arrow: Fistula formation between a vessel and the tracheobronchial tree. White arrows: contrast leakage from the vessel drawing the bronchial tree bronchography. Circle: tortuous and dilated superior division of left bronchial artery.
We have presented a case of massive hemoptysis due to bronchovascular fistula in a patient with a smoking history. An exhaustive diagnostic evaluation was performed with laboratory tests, bronchoscopy, computed tomography and echocardiogram, but the etiology was not found. However, bronchial arteriography showed right and left bronchial tortuous and dilated arteries and a bronchovascular fistula was demonstrated in vivo to be the origin of the patient's hemoptysis.
Dieulafoy's disease is a vascular anomaly characterized by the presence of a tortuous dysplastic artery in the submucosa. The French surgeon Georges Dieulafoy first described it in 1898 as a cause of gastrointestinal bleeding in the stomach,7 but it has been identified in other parts of the body, including the respiratory tract.8 In fact, bronchial Dieulafoy's disease is extremely rare, and it is still unknown whether the origin of the anomaly is congenital or acquired, though age and tobacco use are thought to have an influence on the occurrence of the disease.9 Furthermore, the trigger factor of the vessel rupture is unknown. On the other hand, although bronchitis is an etiology of hemoptysis, it is controversial whether this entity alone is sufficient to cause massive hemoptysis and many clinicians believe that other contributing factors must be present for massive hemoptysis to occur in the setting of bronchitis alone.2 Menchini et al. have reported a retrospective cohort of 35 patients with smoking-related bronchopulmonary disease and no associated comorbidity, who were referred for embolization to cease hemoptysis. Bronchial artery angiography revealed moderate or severe hypervascularization in 28 (80%) patients and no statistically difference was observed between the angiographic findings and the severity of COPD, tobacco consumption or the amount of bleeding.10 He then suspected of the Dieulafoy's disease in these cases. Therefore, recurrent and massive hemoptysis caused by hypervascularization within the bronchial wall due to chronic airway inflammation in the context of a smoking history might be explained by an acquired form of this entity.
Regarding the diagnosis of Dieulafoy's disease of the bronchus, the bronchoscopy may show a small, sessile, non-pulsating nodular lesion, often with a white cap, and apparently normal mucosa.8 At times, the bronchoscopic appearance is not diagnostic because the site where the abnormal vessel opens into the bronchus is usually a pinpoint mucosal defect surrounded by normal-appearing mucosa and this small defect is often not seen on bronchoscopy due to pooling of blood or filling of the bronchial lumen with clots. This is what occurred in our case. Bronchial Dieulafoy's disease can only be definitively diagnosed by histopathologic examination11; however, bronchial biopsy in such cases can result in severe hemorrhage.12 Morover, performing biopsy would be not useful in this context, because the diagnosis of Dieulafoy's disease of the bronchus should be based on the pathological examination of a large surgical lung resection.9 Endobronchial ultrasound may be helpful in detecting the vascular nature of the lesion,13 but this was not available at our hospital. Hope-Gill et al. recommend the bronchial arteriography as the most appropriate initial investigation in these cases.14 There are no specific angiographic criteria for diagnosing Dieulafoy's disease, but the finding of a tortuous and ectatic artery is suggestive of this condition. Selective embolization has been proposed as a method for stopping the bleeding,14,15 and only in a few cases does the patient require surgical resection.4 Recently, Dalar et al. have proposed the argon plasma coagulation of these lesions.16
We have described a peculiar case of massive and recurrent hemoptysis without associated comorbidity, except smoking. The tortuous and dilated bronchial arteries and bronchovascular fistula that we found in the context of hypervascularization might be an acquired form of the Dieulafoy's disease, which would explain the magnitude of bleeding.10 The occurrence of this fact during the bronchial artheriography is extremely rare. To the best of our knowledge, an image of such active bleeding through the bronchovascular fistula has never previously been demonstrated.
ConclusionNowadays, Dieulafoy's disease should always be included in the differential diagnosis of any patient with massive and recurrent hemoptysis. We then considered this possibility given the absence of comorbidity in our patient, except for a smoking history. Since tobacco consumption long-term causes hypervascularization within the bronchial wall, secondary to chronic airway inflammation in the setting of bronchitis, we believe that this disease could be more common than the reports in the literature suggest. Although excision of the affected portion of lung is the gold standard for the definitive histopathologic diagnosis of Dielafoy's disease, surgery is only the option in recurrent and uncontrolled cases. Bronchial arteriography as diagnostic method should be the preferred choice rather than bronchoscopy when the Dieulafoy's disease is suspected, in order to avoid hazardous and unnecessary biopsy procedures altogether.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.