Transbronchial cryobiopsies has become increasingly important in the diagnostic workup for interstitial lung diseases. The rate of complications and mortality are low compared to surgical lung biopsies, but the diagnostic yield is not as high. The reason for the lower diagnostic yield could in some cases be explained by biopsies taken too centrally or in less affected areas. In this pilot study we examined the feasibility of using the electromagnetic navigation system, superDimension (SD), when performing cryobiopsies to increase the diagnostic yield.
MethodsElectromagnetic navigation bronchoscopy and cryobiopsies were performed using SD. An electromagnetic board placed on the back of the patient and a position sensor at the tip of the navigational probe created a real-time 3D reconstruction of previously acquired computer tomography images. The procedure was performed with the patients in general anesthesia using a rigid bronchoscope when performed in Florence and with a flexible bronchoscope through an orotracheal tube when performed in Aarhus.
ResultsIn total, 18 patients were included. Five patients were excluded, partly due to technical difficulties. Disposable 1.7 mm cryoprobes were used in Aarhus, and reusable 1.9 mm probes in Florence. Pneumothorax was detected in three (23%), mild hemorrhage was seen in one (8%) and moderate hemorrhage in six (46%). The biopsies contributed to the diagnosis in 11 of the patients (85%).
ConclusionUsing superDimension electromagnetic navigation system when performing cryobiopsies is feasible. A larger prospective trial is necessary to homogenize the technique between centres and to evaluate diagnostic advantage and complications.
Transbronchial cryobiopsies (TBCB) have gained increasing interest in the diagnostic workup of interstitial lung diseases where lung tissue is needed for a confident diagnosis. Compared to surgical lung biopsies, the procedure is associated with fewer complications, morbidity and mortality. The diagnostic yield of TBCB is not as high, but still close to that of surgical lung biopsies (75-85% vs. 90-98%).1–6
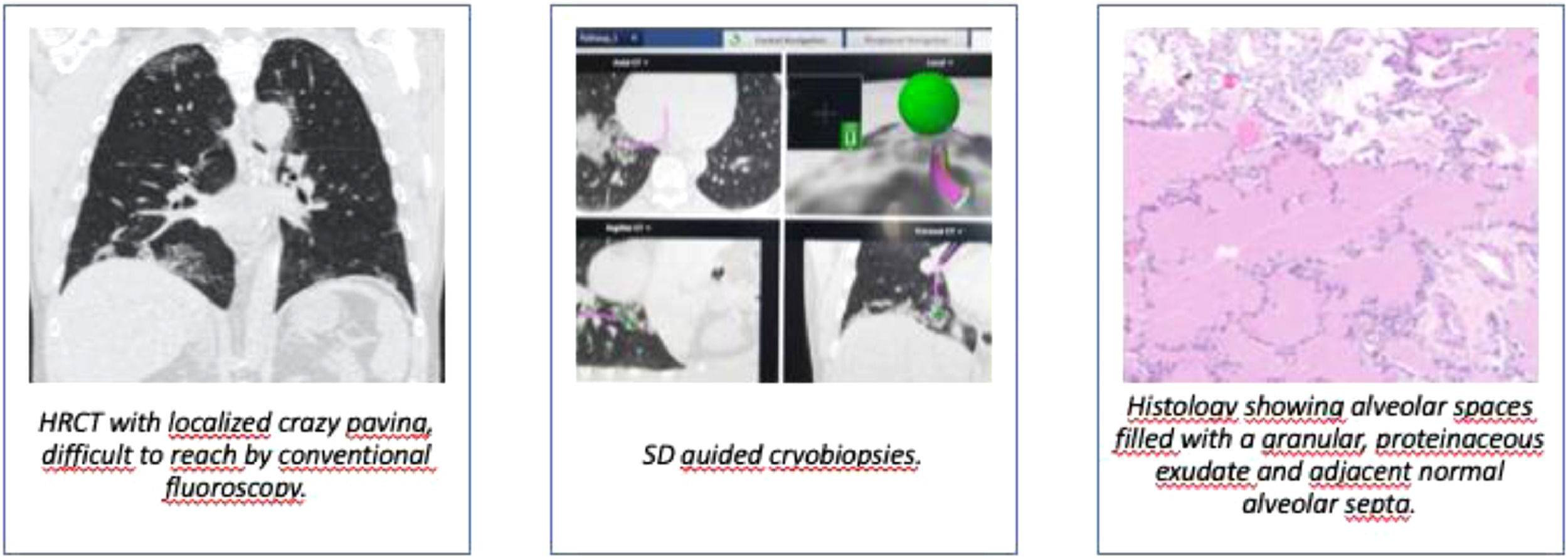
At the moment, most centers use high resolution computed tomography (HRCT) to identify the optimal site for biopsy and fluoroscopic guidance to reach the designated biopsy site. The optimal location is app. 10 mm distance from, and with a perpendicular relation between the probe and the thoracic wall. When biopsies are taken from the lateral parts of the lower lobes, it is relatively easy to ensure correct perpendicular position of the cryoprobe by fluoroscopy. However, when the biopsies are taken from the posterior parts of the lower lobes, in the middle lobe/lingula or in the upper lobes, it is more difficult to assess if the probe is in the right position. In approximately 10-25% of all TBCB, a confident pathological pattern is not obtained.2,5,6 The reason behind the lack of diagnostic yield is multi-factorial but a biopsy that is taken in a central part of the lung and including mainly conducting airways or in a less affected non-representative area of the lung are the main causes. There are many unexplored options combining the TBCB procedure with navigation systems. Some of these are already being used as tools together with the bronchoscope in other contexts, for example electromagnetic based navigated bronchoscopy or ultrasound guided biopsies in the diagnosis of peripheral lung lesions.7,8 The superDimension electromagnetic navigation system is a technology that can be used together with bronchoscopy and enables real-time tracking of the navigational probe and thus provides accurate guidance to the desired area from where to perform the biopsy. It is a system developed for navigation to peripheral lesions in the lungs that are difficult to reach. For the procedure, three devices are needed; a planning software that converts the computed tomography (CT) scan into multiplanar images to reconstruct three-dimensional images of the airways; a steerable probe that contains the position sensor and an electromagnetic board connected to the computer containing the planning data.
A similar technique to guide the bronchoscopist to the optimal biopsy segment in suspected ILD patients undergoing TBCB, would be of great value, especially when the areas of interest are located in difficult to reach parts of the lungs using only fluoroscopy or when there are only subtle changes. Also, this technique could result in a higher diagnostic yield and prevent some patients from having to go through a second TBCB procedure or surgical lung biopsies.
The aim of this pilot study was to explore the feasibility and safety parameters using superDimension system when performing cryobiopsies.
MethodPatients referred for TBCB as part of an investigation for interstitial lung diseases at the Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark and the Department of Experimental and Clinical Medicine, Careggi University Hospital, Florence Italy where included in this pilot study. Eligibility criteria for TBCB have previously been described.6 Baseline demographics regarding gender, age, smoking history, lung function parameters (forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLco)), 6-minute walk test distance and lowest saturation were recorded.
All patients had HRCT performed within four weeks before the procedure. The optimal place from where to take the biopsy was discussed at a multidisciplinary team conference with participation of radiologists and pulmonologists. Patients were eligible for inclusion if any of the biopsies were to be obtained from other areas than the lateral parts of the lower lobes. A compact disc of the scan was created for the superDimensionTM navigation system (ver. 6.0 (Florence) and 7.1 (Aarhus), Medtronic Inc.).
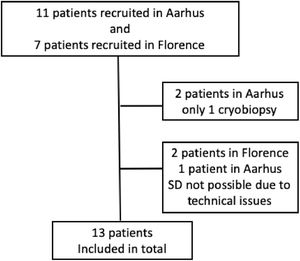
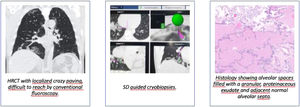
ProcedureBefore the procedure, an electromagnetic board was placed on the back of the patient. The procedure was performed with the patients in general anesthesia intubated with a rigid tracheoscope (Stortz 14) when performed at Careggi University Hospital, Florence, Italy and with a flexible bronchoscope (Olympus, Tokyo, Japan), the patient being intubated with an orotracheal tube, when performed at Aarhus University Hospital, Aarhus, Denmark. All patients were monitored with oxygen saturation, blood pressure, transcutaneous carbon dioxide partial pressure and ECG during the procedure. Cryprobes from ERBE, Tubningen, Germany, were used in both centers. Bronchoalveolar lavage was performed in all patients. Subsequently, a position sensor at the tip of the navigational probe was introduced into the airways through an Extended Working Channel (EWC). In the electromagnetic field generated, the position of the sensor was calculated in real time and merged with the previously created 3D reconstruction of the patient and the place from where to take the biopsy could be found. A Fogarty balloon was then placed at the entrance of the segmental bronchus, ready to be inflated, once the biopsy was obtained to prevent bleeding. In Florence, fluoroscopy was also used right before the biopsies were taken, to make sure the probe was in the right perpendicular position beneath pleura. The cryoprobe was cooled with CO2 for five to eight seconds and then retracted with the frozen lung tissue attached at the tip of the probe. Four biopsies were obtained when possible. The frozen biopsies were thawed in isotonic saline and fixed in formalin and embedded in paraffin, following standard procedure.
Hemorrhage was categorized as mild if only suction was required, as moderate if installation of saline water or extra occlusion of the Fogarty balloon were required and severe if the patient became hemodynamic or respiratory unstable, or if transfusion, surgery or intensive care was needed.
Chest x-ray was routinely performed two hours after the procedure to screen for pneumothorax.
TBCB target, lobe and/or segment, size of cryo-probe, seconds of freezing, complications, and number of biopsies were recorded. All TBCB samples were discussed in a multidisciplinary setting with radiologists, pathologists and pulmonologists.
EthicsThe Regional Ethics Committee for Clinical Trials of the Tuscany Region approved the study (Identifier: 19916_oss). Approval from The Central Denmark Region Committees on Health Research Etichs was waivered upon request.
StatisticsThe results are presented as mean ± standard deviation when normally distributed or as median with interquartile ranges when not normally distributed.
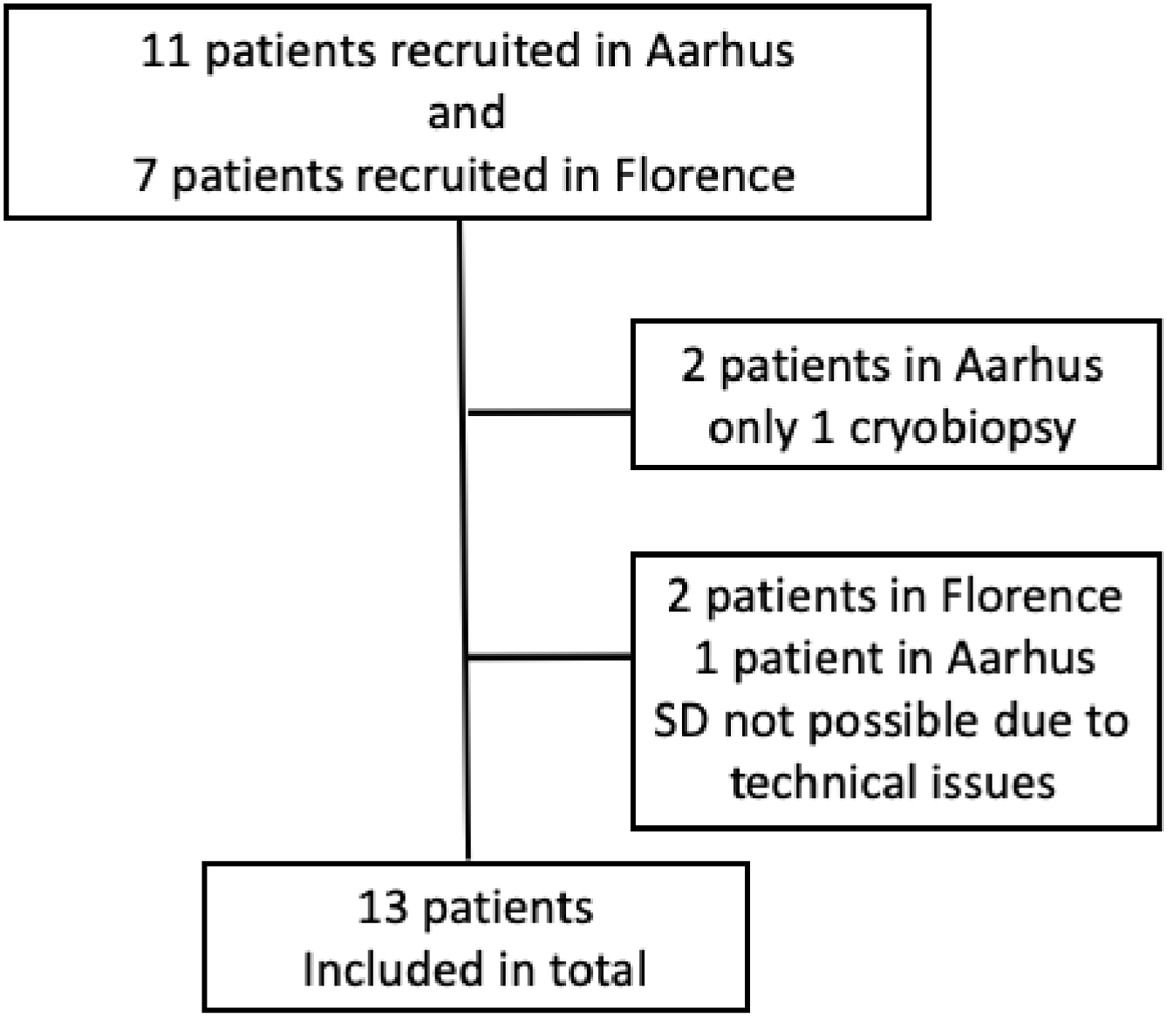
ResultsEighteen patients (11 patients from Aarhus, seven patients from Florence) were recruited and five patients were excluded due to technical difficulties leaving thirteen patients for analysis (Fig. 1). All patients that were excluded had biopsies taken from other areas, but were not included in the analysis. Baseline demographics can be seen in Table 1.
Patient demographics.
In the two first patients in Aarhus, a reusable cryoprobe of 1.9 mm was used and due to the time-consuming procedure, SD was only used for the first biopsy, and subsequently conventional fluoroscopy was used. These patients were not included in the analysis. After this, a disposable cryoprobe of 1.7 mm was used. In Florence, reusable cryoprobes size 1.9 mm were used in all procedures. SD was not possible in two of the patients because of difficulties in getting into the selected segment due to severe fibrosis (one patient in Florence and one in Aarhus) and in one patient due to mismatch between the navigation system and the bronchial tree (Florence). In 12 (92%) patients, biopsies were taken from the right lung (upper lobe: three, middle lobe: three, lower lobe: six) and in one patient, the biopsies were taken from the left lower lobe. Pneumothorax was detected in three of the 13 patients (23%) of which one had a chest tube inserted that was removed the following day (Table 2). Mild or moderate hemorrhage was seen in 7 (54%). None of the patients experienced severe hemorrhage or acute exacerbations. Pleura was not present in any of the biopsies from Aarhus and in Florence, these data were missing. The biopsies contributed to the diagnosis in 11 of the 13 patients (85%). The results from the two sites are presented in Table 2.
Procedure related data and results.
IQR: interquartile range, F: female, M: male, ILD: interstitial lung disease.
We have in this pilot study, as the first, shown that using SD electromagnetic navigation system when performing TBCB with both rigid and flexible bronchoscopes in the diagnostic workup for interstitial lung diseases is feasible.
Performing TBCB with conventional fluoroscopy, even when guided by HRCT scans, is a blind procedure and the biopsies are not always diagnostic. Using the SD system may result in more patients having representative biopsies, a higher diagnostic yield and fewer patients being referred for new TBCB or surgical lung biopsies. In particular, in patients with only subtle changes on HRCT or in patients with changes that are more difficult to reach solely guided by fluoroscopy, the use of the SD navigation system could be beneficial, see Fig. 2. But, like all other procedures, the use of electromagnetic navigation bronchoscopy requires training, both in planning the procedure, using the software and navigating with the multiplanar CT images.
In both centers, the cryobiopsies were performed by experienced interventional pulmonologist that were already familiar with performing TBCB. In Aarhus, TR, was also familiar with using SD for retrieving tissue samples from pulmonary lesions. Even with experienced physicians, the perception was that the procedure took longer time compared to solely using conventional fluoroscopy.
In the first two patients in Aarhus, a cryoprobe of 1.9 mm was used but the procedure was time- consuming due to difficulties in navigating the cryoprobe through the EWC, and thereafter, the new disposable 1.7 mm cryoprobe was introduced for all patients.
The rate of pneumothorax was comparable to other studies using fluoroscopy guided TBCB, but the rate of hemorrhage was higher.6,9 More Danish patients experienced moderate hemorrhage (63%) probably due to more biopsies being central compared to Florence. In Florence, fluoroscopy was used after finding the targeted area and before taking the biopsies, to make sure the probe was in the desired area beneath pleura and thus probably more peripheral. The changes found on CT in the Aarhus cohort were more pronounced more centrally which could also explain the higher rate of bleeding. Also, pleura was not found in any of the Danish biopsies, supporting that the bleeding rate was due to biopsies being more central.6 The perception of the degree of bleeding can be different when using a rigid bronchoscope and a flexible fiberscope. One drop of blood may block the view when working with the fiberscope while it requires more bleeding to interfere when using the rigid bronchoscope. This might influence the labeling of degree of bleeding. As in other studies with TBCB, we found a high diagnostic yield of 85%.5,6,9
Apart from the hardware and software for the SD, a sensor probe at the cost of approximately 1000 Euro is needed for each patient. When using conventional fluoroscopy as guidance when performing TBCB, approximately 10-25% of the biopsies are non-diagnostic. Many of these patients will have to go through a new TBCB procedure or for some, a surgical lung biopsy which will also pose a considerable cost when staff, equipment and possible hospitalization are calculated.
This study has several limitations. As this is a pilot study, the sample size is small and it is not a randomized and controlled trial comparing the diagnostic yield and risk of complications to patients having TBCB performed only with conventional fluoroscopy. Also, the study was not blinded when discussed at MDT meetings. The procedure was performed slightly different in the two centers making it more difficult to compare the results and the use of fluoroscopy might be the reason for more peripheral biopsies and less hemorrhage in the Italian patients. Based on the results in this study, we can not recommend or disadvice against the use of fluoroscopy at the same time as the electromagnetic navigation system when performing TBCB.
ConclusionUsing superDimension when performing cryobiopsies is safe and feasible. Larger randomized, controlled, single blinded multicenter trials are necessary to homogenize the technique between centres and to evaluate diagnostic yield and complications.
Funding informationThe project has been funded by the Central Denmark Region (13 500 Euro) and The Froelich Fund (9 500 Euro). The funding sources were not involved in study design, collection of data, analysis, interpretation of data, writing the report or the decision to submit the article for publication.
The authors do not have any competing interests to declare regarding this study.
The authors do not have any competing interests to declare regarding this study.