Non-small cell lung cancer (NSCLC) is the most common type of lung cancer with a high mortality rate and poor prognosis. miR-637 has been reported to regulate tumor progression and act as a prognosis biomarker of various cancers. Its functional role in NSCLC was investigated in this study.
MethodsThe expression level of miR-637 in NSCLC tissues and adjacent normal tissues of 123 NSCLC patients was analyzed by qRT-PCR. The association between miR-637 and clinical pathological features in the prognosis of patients was analyzed. Cell transfection was performed to overexpress or knockdown miR-637 in H1299 and HCC827. The proliferation, migration, and invasion of H1299 and HCC827 were evaluated by CCK8 and Transwell assay.
ResultsmiR-637 expression was significantly decreased in NSCLC tissues and cell lines relative to normal tissues and cells. The survival rate of NSCLC patients with low miR-637 expression was lower than that of patients with high miR-637 expression. Additionally, miR-637 served as a tumor suppressor that inhibited cell proliferation, migration, and invasion of NSCLC.
ConclusionDownregulation of miR-637 in NSCLC was associated with TNM stage and poor prognosis of patients and served as a tumor suppressor in NSCLC. These results provide a potential strategy to control NSCLC.
Non-small cell lung cancer (NSCLC) accounts for most cases of lung cancer; it is one of the most frequently diagnosed cancers and the leading cause of cancer-related death.1 In clinics, the majority of NSCLC cases have developed to the middle or advanced stage when specific symptoms are apparent, which results in the high mortality rate.2,3 Tumor metastasis, recurrence, and treatment failures are the main factors responsible for the poor prognosis of NSCLC.4,5. Despite the great improvement in the diagnostic and therapeutic strategies of NSCLC in the past decades, the 5-year overall survival rate of NSCLC was still lower than many other cancers. Thus, there is a need for more efficient strategies to improve the prognosis and therapy for patients with NSCLC.
MicroRNAs (miRNAs) are endogenous conserved non-coding RNAs, which can regulate mRNA translation and stability by binding 3′UTR of targets.6,7 Research has shown that miRNA is involved in almost the whole process of tumor occurrence and development. Recently, with the development of molecular biology evidence suggested the modulator role of miRNAs in the differentiation, proliferation, apoptosis, and progression of several cancers.8,9 MiR-340–5p was identified as tumor promoter of thyroid cancer, which promoted thyroid cancer proliferation by inhibiting BMP4.10 miR-153 was considered a prognostic biomarker of prostate cancer, as its upregulation predicates the poor prognosis of patients.11 In NSCLC, downregulated miR-940 has been demonstrated to suppress tumor progression and is associated with poor survival rate of NSCLC patients.12 MiR-512–5p, miR-191, miR-1247, and many other miRNAs have been reported as playing vital roles in the progression and prognosis of NSCLC.13-15 miR-637 is identified as a downregulated miRNA in NSCLC and has been reported to affect the development of various cancers.16 For example, miR-637 was found to be correlated with poor prognosis of glioma and its downregulation could promote tumor cell migration and invasion.17 Moreover, it is also involved in the progression of hepatocellular carcinoma, melanoma, pancreatic ductal carcinoma, and cholangiocarcinoma.18-21 Therefore, the dysregulation of miR-637 might also act as a biomarker for the progression and prognosis of NSCLC. To verify this hypothesis, we investigated the expression and function of miR-637 in NSCLC tissues and cells by a series of in vitro experiments, aimed to provide more references for the treatment and management of NSCLC.
Materials and methodsPatientsNSCLC tissues and adjacent normal tissues of 123 patients with NSCLC were collected during surgery. Patients were recruited from January 2011 to December 2013, which were diagnosed and confirmed with NSCLC at Binzhou Medical University Hospital. All patients had never received any other treatments before surgery. All patients signed informed consent and were followed up for 5 years to obtain the survival information. Collected tissues were confirmed with pathology diagnosis following the International Union against Cancer (UICC) and frozen in liquid nitrogen and stored at −80 °C for the following experiments. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Binzhou Medical University Hospital (NO.: 20101201) and individual consent for this retrospective analysis was waived.
Cell culture and transfectionH1299, HCC827, H1755, and A549 and normal lung epithelial cell BEAS-2B were purchased from ATCC. All cells were cultured in DMEM culture medium with 10% fetal bovine serum (FBS) at 37 °C in a humidified incubator with 5% CO2.
Transfection of miR-637 mimic, miR-637 inhibitor and corresponding negative control (mimic NC and inhibitor NC) (RiboBio, Guangzhou, China) into NSCLC cells was performed with a concentration of 50 nM to regulate the expression of miR-637. Transfection was conducted with the Lipofectamine 2000 Reagent (Invitrogen, USA) following the manufacturer's instructions and evaluated by the expression of miR-637 after transfection for 48 h. Untreated cells were used as mock group.
Quantitative real-time polymerase chain reaction (qRT-PCR)Total RNA was first extracted from collected tissues and cell lines with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Then cDNA was synthesized using a microRNA reverse transcription kit (Applied Biosystems, Foster City, USA). Finally, qRT-PCR was performed to detect the expression of miR-637 with the SYBR Green I Master Mix kit (Invitrogen) and the 7300 Real-Time PCR System (Applied Biosystems, USA). miR-637 expression normalized by U6 and calculated by the 2−ΔΔCt method.22. The thermocycling conditions were as follow: 95 °C for 5 min, 40 cycles of 95 °C for 10 s, 60°C for 20 s, and 72°C for 10 s, and then final extension at 72°C for 5 min.
CCK8 assayTransfected cells were added to the 96-well plates at a density of 5 × 103 cells per well and cultured for 24 h, 48 h, 72 h. At the appropriate time points, CCK8 reagent was added to each well and further incubated for 4 h at 37 °C with 5% CO2. Finally, the absorbance of each well at 450 nm was measured by a microplate reader (Thermo Fisher Scientific).
Transwell assay1 × 105 cells were seeded into the upper chamber of 24-well transwell chambers (8 μm pore size, Multiskan MK3, Thermo, Waltham, MA, USA) incubated with culture medium without FBS at 37 °C for 24 h. In the bottom chamber, the culture medium with 10% FBS was added as a chemoattractant. For the invasion assay, the Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) was added into the upper chamber before seeding cells. The plates were incubated at 37 °C for 48 h, then, migrated and invasive cells were stained with 0.1% crystal violet and counted by a microscope. The number of migrated and invasive cells was obtained from five representative field and calculated the mean value.
Statistical analysisData were presented as mean ± standard deviation (SD) and analyzed by SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software, Inc., Chicago, USA). Comparison between groups was conducted by paired Student's t-test and one-way ANOVA followed Turkey test. Kaplan-Meier analysis and Cox regression analysis were used to plot the survival curves of patients and evaluate the prognostic value of miR-637. P value less than 0.05 was considered statistically significant.
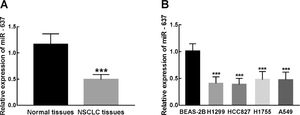
ResultsmiR-637 was significantly downregulated in NSCLC tissues and cell linesThe results of qRT-PCR showed that relative expression of miR-637 in 123 collected NSCLC tissues significantly decreased in comparison with that in adjacent normal tissues (P < 0.001, Figure 1A). The downregulation of miR-637 was also found in NSCLC cells (H1299, HCC827, H1755, and A549) relative to the expression in the normal lung epithelial cell BEAS-2B (P < 0.001, Figure 1B). The dysregulation of miR-637 indicated its potential biomarker role in the prognosis and progression of NSCLC.
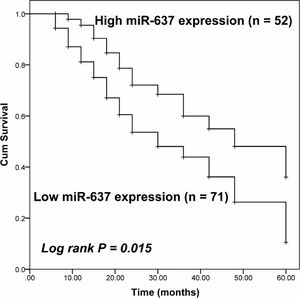
miR-637 was associated with the disease progression in NSCLC patientsAssociation between miR-637 expression level in NSCLC and the clinical and pathological features of patients was evaluated. The average expression level of miR-637 in NSCLC tissues 0.493 was used as the cut-off to divide 123 patients into two groups, including a low miR-637 expression group contains 71 patients and a high miR-637 expression group contains 52 patients. The χ2 test showed that the expression of miR-637 was significantly associated with the TNM stage of patients (P = 0.031), while other features, such as age, gender, differentiation, tumor size, lymph node metastasis, and histology showed no significant association (P > 0.05, Table 1).
Patients’ clinical features and their association with miR-637 expression.
TNM: Tumor Node Metastasis.
Considering the significant association between miR-637 expression and the TNM stage of patients, it was speculated that the downregulation of miR-637 might affect the prognosis of patients. The survival of NSCLC patients was plotted by the Kaplan-Meier method and compared by the log-rank test shown in Figure 2. The decreased expression level of miR-637 was associated with the poor prognosis of NSCLC patients (Log-rank P = 0.015). Additionally, by Cox regression analysis, miR-637 (HR value = 2.234, 95% CI = 1.203–4.149, P = 0.011) and TNM stage (HR value =1.805, 95% CI = 1.026–3.175, P value = 0.040) were considered as two independent risk factors for the prognosis of NSCLC patients (Table 2).
Association between survival rate of patient and patients’ clinical features by Cox regression analysis.
TNM: Tumor Node Metastasis.
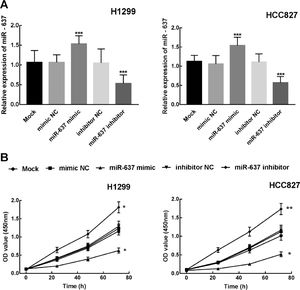
miR-637 was overexpressed or knockdown in H1299 and HCC827 by the transfection of miR-637 mimic or miR-637 inhibitor. The expression level of miR-637 was significantly increased in miR-637 mimic treated cells and decreased in miR-637 inhibitor-treated cells, which indicated the transfection was successful for the following experiments (P < 0.001, Figure 3A).
Next, the proliferation ability of transfected cells was evaluated by CCK8 assay. After the transfection of miR-637 mimic, the proliferation of H1299 and HCC827 was significantly inhibited by the overexpression of miR-637 (P < 0.05, Figure 3B). On the contrary, the downregulation of miR-637 by the transfection of miR-637 inhibitor dramatically promoted NSCLC cell proliferation (P < 0.05, P < 0.01, Figure 3B).
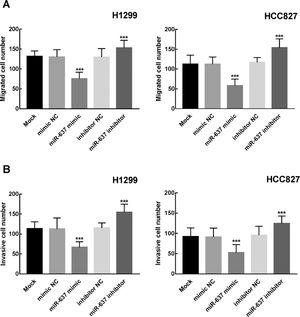
miR-637 inhibited cell migration and invasion of NSCLCThe results of Transwell assay showed that the number of migrated and invasive cells significantly decreased in H1299 and HCC827 after miR-637 mimic transfection, while miR-637 inhibitor promoted cell migration and invasion, indicating the inhibitor role of miR-637 in cell migration and invasion of NSCLC (P < 0.001, Figure 4A and B).
DiscussionNSCLC is the most common pathological type of lung cancer and has been linked to poor prognosis with a bad 5-year survival rate.23,24 MiRNA is the specific kind of RNA that is the focus in the study of the pathogenesis of disease at present25. Recent research has focused on miRNAs and other molecules as a promising approach to regulate disease progression and predicate the prognosis of various cancers.26 In gastric cancer, miR-338–3p, a downregulated miRNA, was identified to predict an unfavorable prognosis of patients.27 miR-340–5p was demonstrated to promote the progression of thyroid cancer by regulating the expression of BMP4, which can be a promising target for the therapy of thyroid cancer.10. miR-541 is a downregulated miRNA in NSCLC, which was reported to inhibit the growth and metastasis of NSCLC cells by targeting TGIF2.28. Moreover, the expression pattern of miRNA has some tissue specificity. For example, miR-590 was upregulated in ovarian cancer and promoted cancer progression,29, while it was downregulated in osteosarcoma and inhibited disease progression by targeting SOX9.30
A number of dysregulated miRNAs in NSCLC have been identified in the previous studies. Notable findings of this study showed the decreased expression of miR-637 in NSCLC tissues and cell lines, which is consistent with the previous result of the miRNA expression profile in NSCLC.16 Deregulation of miR-637 has recently been observed in many other human cancers. Decreased expression of miR-637 was observed in cholangcarcinoma and the overexpression of miR-637 caused a significant increase in the proliferation and migration ability of cholangcarcinoma cell QBC939.21 Downregulation of miR-637 was reported to be associated with the TNM stage, which indicated that miR-637 might be involved in the disease progression of NSCLC.
The prognosis prediction could provide basis for adjusting therapeutic strategies. The prognostic value of miR-637 has been demonstrated in glioma that reduced expression of miR-637 was associated with the poor prognosis of patients and identified as an unfavorable prognosis marker in glioma.17 Here, the obtained survival information of NSCLC patients showed that downregulation of miR-637 predicates the poor prognosis of patients, which makes it act as an independent prognostic indicator for NSCLC. Previous studies have reported the potential biological function of miR-637 in various cancers. For example, miR-637 was considered as a suppressor of hepatocellular carcinoma by regulating signal transducer and activator of transcription 3 (Stat3).18 Another study demonstrated that miR-637 could inhibit cell proliferation and induce apoptosis of human pancreatic ductal adenocarcinoma by targeting Akt120 Several studies have focused on the identification of prognostic biomarkers of NSCLC, but the statistical validation alone was not sufficient.31-33 The prognosis of patients is associated with the disease development. The role of miR-637 in the development of NSCLC was further explored. The expression of miR-637 was regulated in NSCLC cells and found that miR-637 acted as an anti-tumor miRNA due to its inhibitory effect on cell proliferation, migration, and invasion of NSCLC.
The neglect of mechanical research is a limitation of this study. Previously, Akt1 has been validated as a direct target of miR-637 in its suppressed effect on cell growth, migration, and invasion of hepatocellular carcinoma and glioma.17,34 It is speculated that Akt1 might also serve as the direct target of miR-637 in NSCLC, which needs to be verified by a deep mechanical investigation. On the other hand, in vivo experiments are also effective methods to assess the biological function of miR-637 in the disease progression of NSCLC in vivo. However, the above findings also provide a direct reference for further studies to some degree.
Based on these findings, miR-637 was identified as a significantly downregulated miRNA in NSCLC, which was associated with the TNM stage and poor prognosis of patients. Further, miR-637 was an independent prognostic indicator for NSCLC patients and acted as a suppressor in the proliferation, migration, and invasion of NSCLC cells. This study provides a novel potential biomarker and therapeutic target for the prognosis and treatment of NSCLC.
FundingNo fund.
Ethics approvalThe study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Binzhou Medical University Hospital (NO.: 20101201).
Consent to participateIndividual consent for this retrospective analysis was waived.
Consent for publicationNot applicable.
Availability of data and materialThe data that support the findings of this study are available from the corresponding author upon reasonable request.