Pneumocystis jirovecii infection occurs almost exclusively in immunosuppressed individuals. Apart from human immunodeficiency virus (HIV), patients with solid tumors, hematological malignancies, solid organ transplant recipients, primary immune deficiencies or prolonged use of corticosteroids or immunosuppressants are all at increased risk of Pneumocystis jirovecii pneumonia (PCP).1,2
Immunotherapy is a fast-growing field and has revolutionized cancer treatment. Like any therapeutic entity it has its own adverse effects3 but it is not usually associated with an increased risk of infection by Pneumocystis jirovecii. Durvalumab is a IgG1 monoclonal antibody that targets programmed death-ligand 1 (PD-L1) expressed in higher-than-normal amounts by some cancer cells. It is approved in stage III non-small cell lung carcinoma (NSCLC) with PD-L1 expression ≥ 1 % as maintenance therapy after chemoradiotherapy.4 We present a case of PCP in a patient without classic criteria for immunosuppression, but on immunotherapy with durvalumab.
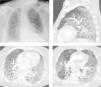
We describe the case of a 66-year-old male patient, with previous history of chronic obstructive pulmonary disease (COPD), former smoker (250 pack-year), arterial hypertension and type 2 diabetes; usually medicated with lisinopril, metformin, aclidinium bromide and budesonide with formoterol. The patient had recently been diagnosed with undifferentiated lung carcinoma (stage IIIA) and underwent 4 cycles of chemotherapy (vinorelbine and carboplatin) and concomitant radiotherapy, followed by durvalumab one month after. One month after initiating immunotherapy the patient presented with dyspnea and productive cough. After 4 days he went to the hospital and was feverish, hypotensive, with SpO2 85 % (FiO2 100 %). Blood gas analysis showed global respiratory failure with severe acidemia (pH 7.16). The patient was admitted into an Intensive Care Unit (ICU) and non-invasive ventilation was initiated but he had an unfavorable evolution, requiring invasive ventilation less than 24 h after admission. He was started on antibiotic therapy with piperacillin/tazobactam and linezolid. Chest CT revealed bilateral ground-glass opacities involving more than 75 % of the parenchyma (Fig. 1). Bronchofibroscopy showed diffuse inflammatory signs and mucopurulent secretions, but microbiological examination of bronchoalveolar lavage was negative. Due to the radiological presentation, a search for Pneumocystis jirovecii by PCR (polymerase chain reaction) was eventually requested, despite the fact that he was an “immunocompetent” individual. The result was positive and the patient was started on targeted therapy with cotrimoxazole. Clinical and radiological improvement was noticed but the hospitalization was prolonged due to multiple complications that included severe acute respiratory distress syndrome (ARDS), refractory shock, need for renal replacement technique and pneumoperitoneum. The patient ended up dying 2 weeks after admission.
Durvalumab seems to increase progression-free survival by 11 months, compared to placebo, in patients with locally advanced unresectable NSCLC.4 Adverse reactions include pulmonary toxicity due to pneumonia (13.1 %) and pneumonitis (12.6 %), although this includes radiation pneumonitis which is expected after chemoradiotherapy. These are mostly low grade reactions and they occur about 1 month after starting therapy, suggesting that monitoring during this period is important.5 Within pulmonary adverse reactions due to Durvalumab, there are very few reports of infection by Pneumocystis jirovecii, one study reporting 5 cases in a total of 2162 adverse event reports5 and another reporting 2 cases in 475 patients.4 In Bauman et al. (CLOVER study) one patient died due to Pneumocystis jirovecii pneumonia,6 which ended up happening in the case presented here.
Symptoms of PCP include fever, cough, chest pain and dyspnea; hypoxia and respiratory failure can occur.1 The radiographic appearance is classically a bilateral interstitial pneumonia with diffuse patchy consolidative and ground-glass opacities,2 although no radiological findings are specific to PCP. When PCP is clinically suspected, bronchoalveolar lavage (BAL) fluid is useful, but it should not delay treatment. In the case described, there were no classic risk factors for the occurrence of PCP but considering the clinical and radiological presentation it was suspected and investigated. This complication of Durvalumab, though rare, is associated with high mortality, so it should be noted and considered as the causal agent.