Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by high red blood cells and splenomegaly, and the vast majority of PV patients harbor the janus kinase (JAK) mutation.1 Ruxolitinib, a potent JAK inhibitor, has been approved as second-line therapy in patients with PV.2 Due to its mechanism of action, ruxolitinib also has immunosuppressive effects. Several opportunistic infections have been reported in patients receiving ruxolitinib, but most data on ruxolitinib-associated infections have been described in patients with primary myelofibrosis (another myeloproliferative neoplasm) and are limited only to single case reports.3 In this document we describe the case of a PV patient treated with ruxolitinib who developed a disseminated tuberculosis mimicking pleural mesothelioma on imaging.
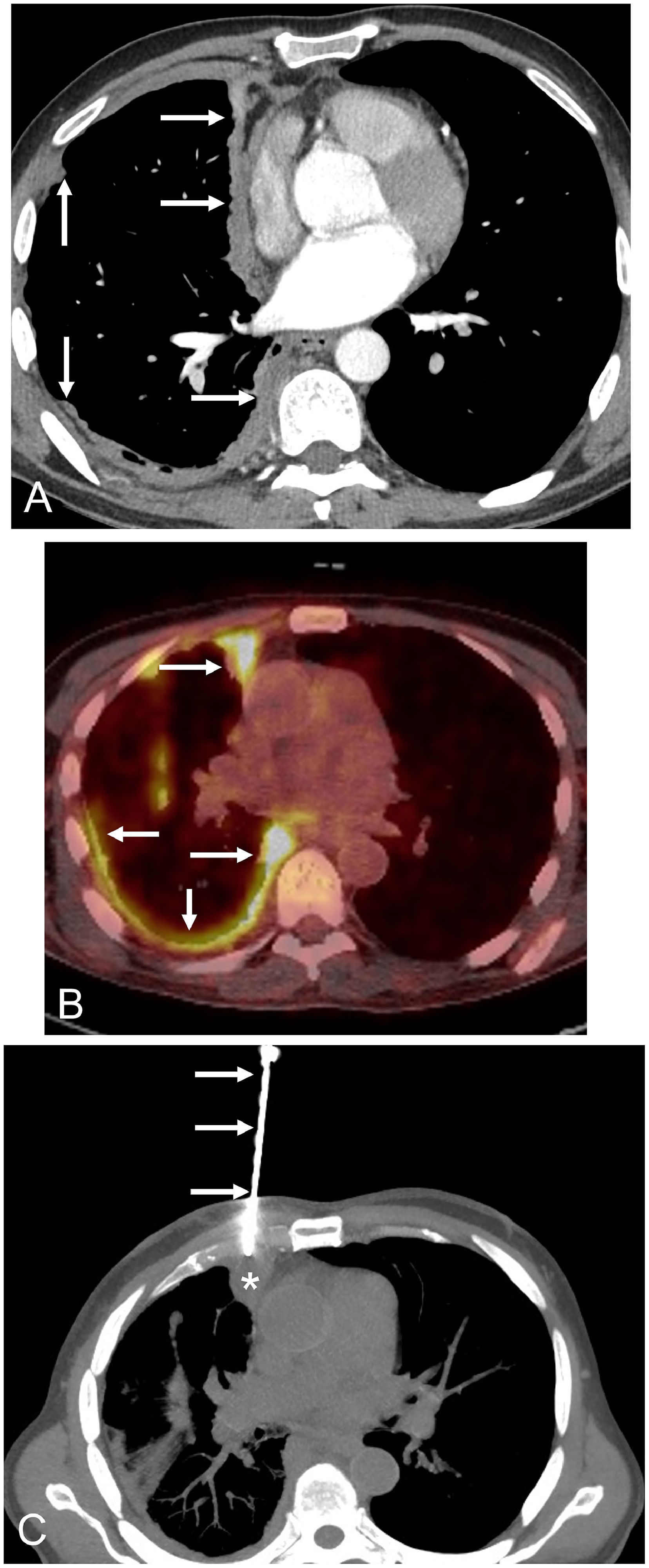
The patient was a 59-year-old non-smoker male without known asbestos exposure, who complained of progressive dyspnea and low-grade fever. The patient was suffering from PV and had started treatment with ruxolitinib 17 months earlier. A chest radiograph showed a marked right lung volume loss with apical pleural thickening. A thoracic computed tomography (CT) revealed a striking nodular thickening of the right pleural surface that particularly involved the mediastinal pleura (Fig. 1A). There were no suspicious lung nodules, enlarged lymph nodes, or significant pleural effusion. A whole-body fluorine-18 fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) demonstrated a markedly increased FDG uptake by the right hemithorax thickened pleural surface (Fig. 1B), but also showed additional foci of hypermetabolic activity involving the peritoneal surface and a single bone lesion in the T5 vertebral body. A presumed diagnosis of a metastatic pleural mesothelioma was made, and the patient underwent a percutaneous CT-guided pleural core-needle biopsy (Fig. 1C). Pathologic findings did not detect cancer cells but revealed extensive necrotizing granulomatous inflammation. Real-time polymerase chain reaction (PCR) for Mycobacterium tuberculosis was detected on one of the percutaneously-obtained specimens, and a final diagnosis of disseminated extranodal tuberculosis was made. The patient was started on four antituberculous drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) and improved both clinically and radiologically.
A) Axial thoracic CT image (mediastinal window) shows a solid nodular thickening (arrows) of the right hemithorax pleural surface that particularly involves the mediastinal pleura. B) Axial fused thoracic PET/CT image demonstrates an intense FDG uptake by the nodular pleural thickening (arrows). C) Axial chest CT image (mediastinal window) shows the biopsy needle (arrows) targeting the nodular pleural surface (asterisk).
Polycythemia vera is a chronic myeloproliferative neoplasm characterized by high hemoglobin levels and splenomegaly because of uncontrolled red blood cell production. A dysregulation in janus kinase (JAK) – signal transducer and activator of transcription signaling pathway is present in the majority of patients with PV.1 Ruxolitinib is a potent agent that inhibits JAK and is indicated in PV patients as second-line therapy (patients who are resistant or intolerant to hydroxyurea). Ruxolitinib is also used in other myeloproliferative neoplasms, such as intermediate or high-risk primary myelofibrosis (PMF), hematologic malignancies, and in steroid-refractory graft-versus-host disease.2 There is increasing evidence that ruxolitinib plays a major role in immunosuppression, since it affects both differentiation and function of dendritic cells, resulting in markedly reduced cytokine production, and decreased expression of costimulatory molecules, halting appropriate antigen-specific T-cell activation and natural killer cells maturation. All these effects may result in severe immunodeficiency, leading to high risks of infectious complications.3,4 Several opportunistic infections have been reported in patients receiving ruxolitinib, including Pneumocystis jirovecii and Cryptococcus neoformans pneumonia, progressive multifocal leukoencephalopathy, disseminated herpes simplex virus infection, hepatitis B virus reactivation, and both pulmonary and extrapulmonary tuberculosis.5 Most data on ruxolitinib-associated infections are available for PMF patients and are limited only to isolated case reports. The lack of ruxolitinib-associated infections in PV patients is due, on the one hand, to the fact that PMF patients per se have higher infection rates and, on the other hand, to the fact that more long-term data are available on the safety of ruxolitinib in PMF patients.6
The presented case represents the first description of disseminated tuberculosis in a PV patient undergoing therapy with ruxolitinib. The atypical imaging presentation of disseminated tuberculosis mimicking pleural mesothelioma hindered the diagnosis of an opportunistic infection in our patient, reinforcing the idea that a high index of suspicion is necessary to correctly diagnose ruxolitinib-associated infections in PV patients. Whole-body FDG-PET/CT was very useful for detecting unsuspected non-pleural infectious lesions in our case. Although there are no specific recommendations in the literature for the prevention of ruxolitinib-associated infections, some researchers are suggesting routine screening, monitoring and/or prophylaxis to avoid complications from these infections in PV patients.7 Even though the incidence of tuberculosis is higher in PMF patients treated with ruxolitinib, a precise medical history should always be taken before starting ruxolitinib treatment in PV, taking into account individual risk factors such as risk groups or travel to endemic areas. It is advisable to perform an interferon gamma release assay (IGRA) or a tuberculin skin test in patients who are going to start treatment with ruxolitinib; if positive, treatment of latent tuberculosis should be strongly considered.3,6