Alpha-1-antitrypsin (AAT) is a glycoprotein synthetized mainly by the liver, acting primarily in the lung as an inhibitor of neutrophil elastase, but also capable of regulating other proteases.1,2
Alpha-1-antitrypsin deficiency (AATD) is a genetic disorder inherited in an autosomal codominant pattern, which is estimated to affect in Europe 1 in 2700 (Northern Europe) to 18,000 (Central Europe).3–5 AATD increases the risk of chronic obstructive pulmonary disease (COPD), emphysema and chronic bronchitis, liver cirrhosis, panniculitis and c-ANCA (anti-neutrophil cytoplasmic antibodies) positive vasculitis, due to a proteolytic imbalance and/or negative effect of protein polymerization.6
Pathogenic mutations in SERPINA1 gene (14q32.13) are the cause of AATD and whereas common M alleles are associated with normal concentrations in the serum, S and Z variants’ have decreased circulating levels of AAT (60% and 15% of the normal values, respectively).1 Most AATD cases are attributed to PI*ZZ genotypes (∼95%) and the remaining ones to PI*SZ, PI*MZ, or any genotype combining rare deleterious mutations.7 To date, there are more than 130 alleles described in the literature, most of them associated with a significant decrease in AAT circulating levels (deficiency variants) or leading to a total absence of protein (null alleles).8,9 One of these variants is the PI*Mmalton (p.Phe52del in a M2 allele) that has been found to polymerize and to accumulate in the endoplasmic reticulum, being secreted into the bloodstream in less than 15% of normal AAT concentration.1 Beside S and Z allele, p.Phe52del (PI*Mmalton or PI*Mpalermo if in a M1alele) appears to be the next most prevalent variant among AATD cases in Iberian Peninsula (54% in Spain and 40% in Portugal).7–10
On the other hand, common variable immunodeficiency (CVID) is a primary immunodeficiency disease characterized by low serum concentration of immunoglobulins which may lead to assorted clinical features,11 mainly recurrent bacterial infections of respiratory and gastrointestinal tracts.12 CVID affects approximately 1 in 30,000 individuals and it is the second most common primary immunodeficiency in humans.12 This disorder is highly heterogeneous and it basically encompasses a group of primary antibody failure syndromes that can be derived from distinct entities all causing some type of hypogammaglobulinemia. To date CVID diagnosis continues to be essentially done by different exclusion criteria.13 Most cases are sporadic and thought to result from a complex interaction between environmental and genetic factors.14 Still, in rare instances, CVID m is inherited in an autosomal recessive fashion, and in other rarer cases, the disease can be inherited in an autosomal dominant pattern.14 Nevertheless, even when CVID is associated with a genetic mutation, additional environmental and genetic risk factors are likely to be necessary for disease onset.14
The advent of clinical manifestations and the detection of low levels of immunoglobulins may occur at any age from early childhood to old age.13 In an European study, 34% of patients presented the disease before the age of 10 years, with male predominance of 2:1 before age 11, and a slight female predominance of 1.3:1 after the age of 30.13
So far, only two cases of subjects combining AATD and CVID have been reported.15,16 Nevertheless, Sansom et al., while studying 43 patients with CVID found five patients with both conditions, suggesting a cumulative effect of AATD and CVID in the presentation of bronchiectasis.17 In these reports, various A1AT genotypes were evaluated, including various combinations of Z with S and M alleles. Concerning the loss of lung parenchyma few studies have attempted to investigate the impact of sharing these respiratory illnesses. Still a previous work proposed a physical correlation between hypogammaglobulinemia and AATD, later confirmed by another study proving the occurrence of genetic linkage between immunoglobulin heavy constant gamma (Gm markers) loci (IGHG1-3 genes) and AAT gene (SERPINA1).15
We present the case of a Portuguese woman, born in Coimbra (Central Portugal) and currently living in Madeira, with history of childhood asthma whose symptoms ceased spontaneously during adolescence. At the age of 32 and having been a tobacco smoker for a decade, the patient was hospitalized for pneumonia with a pleural effusion. Afterwards, respiratory symptoms, such as dyspnea and cough with sputum production become recurrent and multiple respiratory infections were diagnosed within a short time period (five years). In 2000, the patient was found to have CVID upon evaluation of immunoglobulins levels by nephelometry. Consequently, she initiated replacement therapy with subcutaneous immunoglobulin (so currently under 19 years of therapy).
At the age of 57, she was tested for AATD. This analysis showed that she had reduced AAT levels in the blood (18.7mg/dL by nephelometry) and the initial genetic screening yielded a MM result. Given that the first genetic diagnosis of AATD was inconsistent with serum levels, a sample was sent to an informal reference center in Portugal (IPATIMUP). This reevaluation comprised AAT phenotyping by isoelectric focusing, genotyping of four polymorphic sites including S and Z mutations and sequencing of SERPINA1 coding region.9 Only then was a conclusive result achieved, which revealed Mmalton homozygosity (p.Phe52del in a M2 allele) as the cause of AATD. At that time, the patient already presented signs of airway obstruction in lung function tests (Table 1). Given that she had been a former smoker for more than six months she began AAT augmentation therapy in August of 2015.
Patient lung function tests. Prior to AAT augmentation therapy in 2015 and current values. Both results are from post-bronchodilation tests.
| Date | FEV1 (L) | FEV1 (%) | FEV1/VC max | TLC (L) | TLC (%) | RV (L) | RV (%) |
|---|---|---|---|---|---|---|---|
| 15/05/2015 | 0.86 | 56.1 | 45.31 | 4.49 | 125.3 | 2.59 | 170.8 |
| 22/11/2018 | 1.37 | 93.9 | 52.98 | 4.77 | 133.2 | 2.18 | 139.8 |
FEV1: forced expiratory volume in the first second; VC max: maximal vital capacity; TLC: total lung capacity; RV: residual volume.
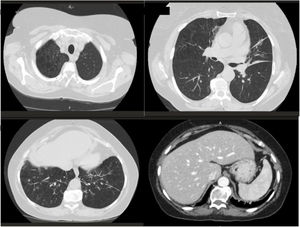
Respiratory complications became less frequent once AAT augmentation therapy started. Still, the patient had on average two exacerbations per year of an infectious nature with a total of seven hospitalizations in four years. After achieving a diagnosis of AATD the patient underwent a chest tomography scan to evaluate the extension of lung disease. This revealed bilateral bronchiectasis, mostly in basal regions, and centrilobular and paraseptal emphysema, mainly in superior lobes (Fig. 1).
Liver disease associated to AATD was also assessed by FibroScan® (measurement of liver stiffness to estimate liver scarring), which revealed a liver stiffness value of 7.9kPa. A score that is compatible with significant fibrosis in the absence of liver cirrhosis.18
This work reports a case of a Portuguese patient diagnosed with two rare diseases: CVID and AATD. This case supports the findings of other authors, confirming the synergetic effect of both conditions on lung disease, leading to the appearance of bronchiectasis. This was well-established by Peppers et al.,19 who found a strong relationship between unresolved or worsened bronchiectasis and lower levels of AAT, and resolved or improved bronchiectasis and higher levels of AAT, when submitting patients with immunodeficiencies under gammaglobulin infusions to serial chest CT scans. The pool of immunodeficient patients selected in their research had a higher prevalence of AAT mutations than the general population, establishing that the determination of the AAT phenotype in these patients is warranted.
In our patient, whereas CVID (by increasing patient susceptibility to pulmonary infections) is likely to have prompted a protease release by inflammatory cells (neutrophils and macrophages), it was AATD impaired anti-elastase activity that probably facilitated alterations in lung structures. Consistently, another previous study comprising a total of 43 patients with CVID in which five were found to have AATD, also suggested an additive effect of these disorders into pulmonary illness.17
Despite so few reports of patients combining CVID and AATD, some studies have attempted to physically correlate these disorders: one of them, performed in 1983, failed to detect any evidence of linkage between these conditions in a family where hypogammaglobulinemia and AATD were found to segregate.16 Interestingly, SERPINA1 encoding AAT is located in chromosome 14 approximately 11Mb apart from a cluster of immunoglobulin genes (IGHG1-3 among others), which could explain in some rare cases a co-segregation of these disorders. However, a genetic screening of CVID was not performed in this case due to its low impact on patient follow up as well as it potential high costs to healthcare system.13
In result of the CVID and AATD joint diagnosis, this patient is currently undergoing two replacement therapies with subcutaneous immunoglobulin and endovenous human AAT. Here, it is important to stress that AAT infusion contains small amounts of IgA. This obligates a careful administration of AATD therapy in patients with IgA deficiency since they present a greater risk of adverse anaphylactic reactions due to the pre-existence of IgA antibodies. The Portuguese consensus document for AATD management actually discourages augmentation therapy in patients with IgA deficiency,20 but so far this patient has been successfully treated with no record of side effects. Furthermore, lung functions tests of this patient have shown a remarkable increase in forced expiratory volume in one second (FEV1) after three years of AAT therapy. Although this phenomenon has not been described before, we believe it might be explained by a combination of optimized inhaled bronchodilators and AAT therapies together with cigarette smoking cessation prior to treatment in 2015 and inter-operator dependent differences in test execution and by the variability in the capacity of performance of the tests by the patient. On the other hand, the finding of liver fibrosis as confirmed by FibroScan® assessment were in accordance with previous studies of Mmalton patients (homozygous and/or heterozygous) showing that this allele is associated with hepatic accumulation of AAT and subsequently with liver disease, being to some degree a functional equivalent to a Z allele.21
Even though we could not rule out a genetic linkage between CVID and AATD in this case, we provide evidence that these might be somehow correlated with observed clinical features of lung disease. This report also emphasizes the singularity of combining two uncommon replacement therapies in controlling the worse outcomes of CVID (recurrent exacerbations) and AATD (severe airflow obstruction).
Conflict of interestsThe authors declare no conflict of interests.
N. Martins would like to thank the Portuguese Foundation for Science and Technology (FCT-Portugal) for the Strategic project ref. UID/BIM/04293/2013 and “NORTE2020 – Northern Regional Operational Program” (NORTE-01-0145-FEDER-000012). IPATIMUP integrates the i3S Research Unit, which is partially supported by the Portuguese Foundation for Science and Technology (FCT; projects PEstC/SAU/LA0003/2013 and POCI-01-0145-FEDER-007274) and by Programa Operacional Regional do Norte (ON.2 – O Novo Norte and Norte 2020), through FEDER funds under the Quadro de Referência Estratégico Nacional (QREN; projects NORTE-07-0162-FEDER-00018 and NORTE-070162-FEDER-000067, and NORTE-01-0145-FEDER-000029).