The Bronchiectasis Health Questionnaire (BHQ) is a simple, repeatable, and self-reporting health status questionnaire for bronchiectasis. This study aims to cross-culturally adapt the BHQ into Brazilian Portuguese and evaluate its measurement properties.
MethodsThe participants answered the Saint George...s Respiratory Questionnaire (SGRQ) and the modified Medical Research Council (mMRC) scale for dyspnea. The Brazilian-Portuguese version of the Bronchiectasis Health Questionnaire (BHQ-Brazil) was used at baseline (test) and after 14 days (retest). The psychometric analyses included internal consistency, test-retest reliability, and construct validity: factorial validity, convergent validity, and discriminative validity, agreement, and ceiling and floor effects.
ResultsThe BHQ-Brazil demonstrated adequate internal consistency (Cronbach...s alpha...=...0.92) and substantial reliability (intraclass correlation coefficient...=...0.86; 95%CI: 0.79...0.90). The exploratory factorial analysis was considered suitable. All items presented a factorial load >0.40. The convergent validity of the BHQ-Brazil with mMRC was moderate (r...=......0.53, p...<...0.001), while concurrent validity with the SGRQ was strong (symptoms: r...=......0.72, activities: r...=......0.60, impact: r...=......0.60, total score: r...=......0.75, all p...<...0.001). The standard error of measurement was 4.81 points. The discriminative validity demonstrated that individuals with more pulmonary exacerbations, colonization by Pseudomonas aeruginosa, worst dyspnea, and a higher number of affected lung lobes presented the lowest quality of life. No floor or ceiling effects were observed.
ConclusionThe BHQ-Brazil presents adequate measurement properties to evaluate the impact of bronchiectasis on health-related quality of life, and can be used in clinical and research settings.
Individuals with bronchiectasis present cough, abundant pulmonary secretion, dyspnea, reduced exercise capacity, and frequent pulmonary exacerbations,1...3 culminating in a worse health-related quality of life.4...6 Quality of life questionnaires provide valuable information regarding the impact of the disease on health perception.7
The Quality of Life Questionnaire-Bronchiectasis (QoL-B) was the first specific questionnaire developed for bronchiectasis.8...10 This questionnaire has the advantage of quantifying the different quality of life domains. However, it is relatively long and does not present a total score, which makes its use disadvantageous in clinical practice. The Saint George...s Respiratory Questionnaire (SGRQ)11...14 and the Chronic Respiratory Disease questionnaire14 were developed for individuals with chronic obstructive pulmonary disease and validated for bronchiectasis since both diseases present common clinical symptoms.11,13...15 These instruments are useful; however, they are extensive and time-consuming. The Chronic Obstructive Pulmonary Disease Assessment Test (CAT) is also a validated questionnaire for bronchiectasis16...18 but it is not specific for this disease, an important requirement that must be considered during the quality of life assessment.19
In this context, the Bronchiectasis Health Questionnaire (BHQ)20 was developed specifically for bronchiectasis and has the advantage of being short, simple to apply and interpret, and generates a total score. Thus, it can be easily implemented during routine clinical evaluations. It is also the first assessment tool addressing items related to pulmonary exacerbation in bronchiectasis, an important marker of quality of life decline.6 In the original, Korean, and Danish BHQ versions,20...22 the exploratory factor analysis (EFA), the standard error of the measurement (SEM), the smallest detectable change (SDC), and the presence of ceiling and floor effects were not investigated. These properties are extremely important when evaluating psychometric properties in quality of life questionnaires. Additionally, the BHQ has been translated into 11 languages but not into Brazilian Portuguese. Therefore, this study aimed to adapt the BHQ to Brazilian Portuguese (BHQ-Brazil) cross-culturally and test its psychometric properties in individuals with bronchiectasis.
MethodsThis is a cross-sectional study approved by the Human Research Ethics Committees of the University of Nove de Julho (number: 2.532.903) and the University of S.·o Paulo (number: 2.574.759). All volunteers agreed to participate and signed an informed consent form.
The study was conducted in two phases. In phase I, the original BHQ was cross-culturally adapted to Brazilian Portuguese following previously established guidelines.23,24 In phase II, the measurement properties of the BHQ-Brazil were tested. In this phase, the consensus-based standards for the selection of health measurement instruments (COSMIN) checklist were used.25 Also based on recommendations,25,26 a minimum sample size of 100 individuals was considered for this study. Additionally, based on the recommendation of 10 participants per item,26 a minimum sample size of 100 individuals was considered sufficient for this study.
The subjects were recruited (convenience sample) by physiotherapists between October 2017 and December 2018 at the Obstructive Diseases Outpatient Clinic of the University of S.·o Paulo Hospital and were sent to the Cardiopulmonary Rehabilitation Center of University of Nove de Julho. Those with a clinical and tomographic diagnosis of bronchiectasis, age ...18 years, and clinically stable (i.e., without coughing, greater volume and/or thicker pulmonary secretion consistency, purulent pulmonary secretions, increased dyspnea, reduced exercise tolerance, greater fatigue, or malaise in the four weeks before the study) were included in the study.27 The exclusion criteria were smoking or tobacco load >10 pack/years, pulmonary (asthma, chronic obstructive pulmonary disease, interstitial lung disease, or cystic fibrosis) or cardiovascular diseases associated, or the inability to answer the questionnaires.
Phase I ..÷ cross-cultural adaptationThe initial translation was done by two independent bilingual translators residing in Brazil, whose native language was Brazilian Portuguese and English as their second language. The two translated versions were compared and combined to produce the first Brazilian version of the BHQ. This version was then back-translated into English by two independent bilingual translators with no prior knowledge and no access to the original version. After this phase, an expert panel composed of two pulmonologists and three physiotherapists compared the original version, translations, and back-translations, and formulated a pre-final version.
The pre-final version was given to a sample of 10 participants to determine whether they understood each item, and the following issue was observed: participants taking long-acting antibiotics demonstrated difficulty in understanding ..úItem 10..Ñ, which addresses the need for antibiotics due to pulmonary exacerbations in the previous 12 months. The panel then decided to cross-culturally adapt this question as follows: ..úIn the last 12 months, I made use of antibiotics to treat an episode of lung infection...Ñ This new version was then given to 30 different individuals, who demonstrated no difficulty in understanding the BHQ-Brazil. The translated version was sent to the instrument developer and approved (Supplementary material).
Phase II ..÷ evaluation of measurement propertiesAfter meeting the eligibility criteria, the participants answered the SGRQ10 and the modified Medical Research Council (mMRC) scale for dyspnea,28 followed by the BHQ-Brazil (test). All questionnaires were administered as an interview form. After 14 days, an evaluation was performed with the same individuals for data collection regarding demographic, anthropometric characteristics, and lung function. During this second visit, the individuals answered again the BHQ-Brazil (retest), which was also administered as an interview form by the same interviewer. The following psychometric analyses were included: reliability (internal consistency and test-retest reproducibility), construct validity (factorial validity and hypothesis-testing), criterion validity (concurrent validity), and agreement.
Testing proceduresBronchiectasis Health QuestionnaireThe BHQ is a specific questionnaire for bronchiectasis and comprises 10 items addressing aspects inherent to the disease. The score ranges from 0 to 100 points, with a higher score indicating a better health status.20
Saint George...s Respiratory QuestionnaireThe SGRQ comprises 50 items and 76 answers, divided into four domains: symptoms, activity, impact, and total. Each item is scored from 0 to 100 points, with higher scores denoting a greater negative impact on the quality of life due to the disease.11
Bronchiectasis Severity ScoresThe severity of the bronchiectasis was evaluated using the E-FACED index (categorized into mild [0...3 points], moderate [4...6 points] or severe [7...9 points])27,28 and the Bronchiectasis Severity Index (BSI) (categorized into mild [0 and 4], intermediate [5 and 8], and severe [>9]).29...31
Data analysisData normality was investigated using the Shapiro-Wilk test, and values were expressed as mean........standard deviation and 95% confidence interval (95%CI). The paired t-test was used to compare BHQ-1 and BHQ-2 scores Effect-sizes were calculated using Cohen...s d.32 The level of significance was set at 5% (2-tailed) for all analyses.
Measurement propertiesReliabilityInternal consistencyThe Cronbach...s alpha was used to calculate the internal consistency for the total BHQ score. Values between 0.75 and 0.95 were considered appropriate.33,34
Test-retest reproducibilityThe type 1 intraclass correlation coefficients (ICC2...1) and 95%CIs were calculated. The following classification was considered: poor (<0.4), moderate (0.4... 0.75), substantial (0.75...0.90), and excellent (>0.90).33,34 Concordance was also analyzed using the Bland-Altman plot.
Construct validityConstruct validity was evaluated using the Factorial Validity and Hypothesis-testing.
Factorial validityThe factorial validity was tested using the EFA, and two methods (Kaiser-Meyer-Olkin [KMO] criterion and Bartlett...s sphericity test) were applied to analyze whether the data matrix could be submitted to factorization. The KMO indices were interpreted as unacceptable (<0.5), mediocre (between 0.5 and 0.7), good (0.7 and 0.8), very good (>0.8), and excellent (0.9).33 The principal component analysis (PCA) with varimax orthogonal rotation was used for data extraction. As the factor analysis aims to reduce the number of variables into fewer numbers of factors, only those factors with an eigenvalue >1 were retained for analysis.35 The factorability of the correlation matrix values were interpreted as minimal (...0.30), important (...0.40), and practically significant (...0.50).36
Hypothesis-testingThe convergent validity was tested using Pearson...s correlation between the BHQ-Brazil and mMRC scores. The correlation coefficient values were interpreted as weak validity (<0.30), moderate validity (...0.30 to <0.60), and strong validity (...0.60).37
The discriminant validity (known groups) analyses whether a measure can discriminate groups in which differences are theoretically expected to be found.37 Then, BHQ-Brazil scores were compared according to the number of exacerbations (0...2 versus 3...6), colonization by Pseudomonas aeruginosa (yes or no), mMRC (0...2 versus 3...4), and the number of affected lung lobes (1...2 versus >2). These dichotomizations were based on E-FACED variables.30 The discriminant validity was performed using the unpaired t-test, as variables exhibited parametric distributions.
Criterion validityPearson...s correlation coefficients were used to confirm the concurrent validity between the total BHQ-Brazil and SGRQ scores.37
AgreementAgreement was analyzed using the SEM (SEM...=...SD...1-ICC), and interpreted as very good (..±5%), good (5%...10%), questionable (11%...20%), and bad (>20%). The SDC was calculated based on the SEM using the following formula: SDC...=...1.96...........2........SEM.
Ceiling and floor effectsCeiling and floor effects were tested by examining the score distribution across participants and considered if 15% achieved the minimum or maximum score on each scale.34
ResultsCross-cultural adaptationThe expert panel performed the cross-cultural adaptation of ..úItem 10..Ñ of the original questionnaire from ..úIn the last 12 months, I needed to take antibiotics for a chest infection..Ñ to ..úIn the last 12 months, I made use of antibiotics to treat an episode of lung infection...Ñ
Assessment of measurement propertiesA total of 103 individuals with bronchiectasis were included; two were excluded due to heart disease. Thus, the final sample consisted of 101 individuals (60 female). None of the patients exacerbated during the study period. Regarding the bronchiectasis etiology, 40% was idiopathic, 24% was due to infection, and other causes 36%. A total of 43% had colonization by Pseudomonas aeruginosa, 13% by Haemophilus influenzae, and 23% by other bacteria, whereas 21% had no colonization (Table 1).
Characteristics of the participants, n...=...101 (60 women).
| Characteristics | Value |
|---|---|
| Age, years old, mean (SD) | 49.0 (14.0) |
| BMI, kg/m2, mean (SD) | 25.0 (4.0) |
| FVC, L, / % pred, mean (SD) | 2.4 (0.8) / 67.0 (17.0) |
| FEV1, L, / % pred, mean (SD) | 1.5 (0.6) /51.0 (18.0) |
| FEV1/FVC, mean (SD) | 62.0 (15.0) |
| O2 dependent, n (%) | 11 (10.9) |
| Number of exacerbations/year, mean (SD) | 1 (0.47) |
| mMRC, mean (SD) | 2 (0.93) |
| n per score 0/1/2/3/4 | 3/39/35/18/6 |
| Pneumectomy, n (%) | 4 |
| E-FACED, mean (SD) | 3 (2.0) |
| n per score mild/moderate/severe | 65/29/7 |
| BSI, mean (SD) | 7 (4.0) |
| n per score low/intermediate/severe | 27/46/28 |
| BHQ -1 time to answer, min, mean (SD) | 3.8 (1.0) |
| BHQ -2 time to answer, min, mean (SD) | 3.6 (0.9) |
| BHQ -1, mean (SD) | 58.8 (8.0) |
| BHQ -2, mean (SD) | 59.0 (9.0) |
| SGRQ symptom, mean (SD) | 55.2 (19.0) |
| SGRQ activity, mean (SD) | 61.0 (20.0) |
| SGRQ impact, mean (SD) | 38.0 (17.0) |
| SGRQ total, mean (SD) | 48.0 (15.0) |
SD: standard deviation, BMI: body mass index; kg/m2: kilograms per square meter; FVC: forced vital capacity; FEV1: forced expiratory volume in first second; L: liters; %: percentage; pred: predicted value; n: number of patients; mMRC: modified Medical Research Council dyspnea scale; E-FACED: exacerbations, forced expiratory volume in first second, age, chronic colonization by Pseudomonas aeruginosa; BSI: Bronchiectasis Severity Index; min: minutes; BHQ: Bronchiectasis Health Questionnaire; SGRQ: Saint George...s Questionnaire.
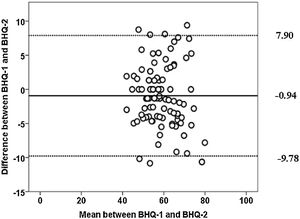
The BHQ-Brazil exhibited adequate internal consistency. The ICC2,1 was considered substantial and demonstrated good test-retest reliability (Table 2). The Bland-Altman plot showed a mean bias of ...0.94, with limits of agreement from 9.78 to 7.90 (Fig. 1).
Classification of measurement properties of Bronchiectasis Health Questionnaire in Brazilian Portuguese in participants with bronchiectasis (n...=...101).
| Properties | Values |
|---|---|
| Reliability | |
| Cronbach...s alpha | 0.92 |
| ICC2.1 (95% CI) | 0.86 (0.79...0.90) * |
| Convergent validity | |
| mMRC | r...=......0.53* |
| Concurrent validity with SGRQ | |
| Symptoms | r...=......0.72* |
| Activity | r...=......0.60* |
| Impacts | r...=......0.60* |
| Total score | r...=......0.75* |
| Agreement | |
| Standard error of measurement | 4.81 points |
| Smallest detectable change | 6.07 points |
| Ceiling and floor effects | Absents |
ICC: intraclass correlation coefficient; CI: confidence interval; SGRQ: Saint George...s Questionnaire; r: Pearson...s correlation, mMRC: modified Medical Research Council dyspnea scale, CI: confidence interval.
For the EFA adequacy, the correlation matrices showed values between 0.40 and 0.90 in most cases. Bartlett...s sphericity test rejected the null hypothesis (p...<...0.001), and the KMO test (0.742) was suitable to proceed to EFA.
Considering the scatter plot graph and using the principal component extraction method with orthogonal varimax rotation, the following three items were identified among the 10 BHQ-Brazil items: (1) tiredness, (2) functionality, and (3) anxiety. Cronbach's alpha coefficients for these items were 0.82, 0.75, and 0.60, respectively. All the items' scores displayed adequate communalities (from 0.60 to 0.79). The initial analysis of the eigenvalues of these three items, after rotation, explained 67.16% of the variance. Thus, as most items presented high factorial loads, the three items extracted could explain the common variance between them (Table 3).
Analysis of the factorial components of each item on the BHQ-Brazil obtained by the varimax rotation method (n...=...101).
| BHQ items | Factors | h2 | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 4. In the last 2 weeks, my chest has felt clear. | 0.76a | 0.40 | 0.761 | |
| 7. In the last 2 weeks, my sleep has been disrupted because of my bronchiectasis. | 0.73 | 0.670 | ||
| 9. In the last 2 weeks, my phlegm (sputum) contained blood. | 0.91 | 0.838 | ||
| 10. In the last one year, I have taken courses of antibiotics for a chest infection. | 0.83 | 0.737 | ||
| 1. In the last 2 weeks, I have been tired. | 0.84 | 0.757 | ||
| 2. In the last 2 weeks, I have been much slower at doing things than other people of my age. | 0.82 | 0.698 | ||
| 3. In the last 2 weeks, I have felt anxious. | 0.43 | 0.604 | ||
| 6. In the last 2 weeks, I have felt short of breath. | 0.87 | 0.792 | ||
| 5. In the last 2 weeks, I have been embarrassed because of my phlegm (sputum). | 0.85 | 0.727 | ||
| 8. In the last 2 weeks, I have had coughing bouts. | 0.75 | 0.623 | ||
| Numbers of items | 4 | 4 | 3 | |
| % of explained variance | 33.68 | 19.96 | 13.52 | |
| % total of explained variance | 67.16 | |||
| Cronbach...s alpha | 0.82 | 0.75 | 0.60 | |
Extraction method: Principal component analysis; Rotation method: Varimax with normalization Kaiser; factor 1, 2, 3.
For convergent validity, moderate correlations were found between the BHQ-Brazil score and mMRC scale (p...<...0.001) (Table 2). The instrument demonstrated good discrimination regarding the number of pulmonary exacerbations, colonization by Pseudomonas aeruginosa, the number of affected lung lobes, and the severity of dyspnea (mMRC) (Table 4).
Discriminative validity of Bronchiectasis Health Questionnaire scores according to exacerbation, colonization, number of lobes affected and dyspnea.
| Variable | Mean (SD)/CI 95% | Mean (SD)/CI 95% | Difference in mean/CI 95% | ES | p Value |
|---|---|---|---|---|---|
| Number of exacerbations in the previous year | 0 ... 2 ( n...=...77) | 3...6 (n...=...24) | |||
| 60.0 (8.25)/58.0...62.0 | 53.0 (6.0)/50.0...55.0 | 7.0/3.7...10.0 | 0.87 | <0,001 | |
| Colonization by Pseudomonas | Not (n...=...57) | Yes (n...=...44) | |||
| 60.0 (8.0)/57.0...62.0 | 56.0 (7.0)/54.0...58.0 | 4.0/0.75...7.24 | 0.50 | <0.001 | |
| Number of lung lobes affected | 1 ... 2 (n...=...35) | > 2( n...=...62) | |||
| 61.0 (8.3)/58.0...64.0 | 57.0 (8.0)/54.0...59.0 | 4.0/0.93...7.8 | 0.50 | =0.01 | |
| mMRC | 0...2 (n...=...77) | 3...4 (n...=...24) | |||
| 60.0 (7.8)/59.0...62.0 | 52.0 (6.2)/49.0...54.0 | 8.0/5.0...12.0 | 1.14 | <0.001 | |
SD: standard deviation, CI: confidence interval, mMRC: modified Medical Research Council dyspnea scale, ES: effect size.
Concurrent validity was demonstrated by strong correlations between the SGRQ domains and the total BHQ-Brazil score (p...<...0.001).
AgreementThe SEM and the SDC of the BHQ-Brazil score were considered very good. No ceiling or floor effects were found (Table 2).
DiscussionThe present study addressed the cross-cultural adaptation of the BHQ-Brazil and the evaluation of its psychometric properties, which were not investigated in the original, Korean, and Danish versions.20...22 The BHQ-Brazil version exhibited adequate internal consistency and substantial reliability when retested after two weeks, presenting values above those recommended for health status questionnaires34 and similar to those reported by the developers of the original, Korean, and Danish versions.20...22 It also exhibited greater internal consistency and reliability compared with CAT.16 The BHQ and CAT are short questionnaires that generate a single final score, which is advantageous for clinical use in bronchiectasis patients.16...18 However, CAT is not specific for bronchiectasis and does not address any pulmonary exacerbation treatment item.
This study also tested the EFA of the BHQ-Brazil, a psychometric property that was not evaluated in the original, Korean, and Danish studies. Suitability was assessed using the KMO and Bartlett tests. The PCA extracted three factors. The first was related to the presence of pulmonary secretion and blood in the secretion, quality of sleep, and pulmonary exacerbation; the second addressed tiredness, functionality, anxiety, and shortness of breath; while the third factor concerned the presence of pulmonary secretion, embarrassment because of phlegm, and cough. The items of each factor presented high factorial load and good internal consistency between them. The communalities of the items were considered adequate, indicating that the scores of the BHQ-Brazil items share a good level of variance. The total variance explained by the BHQ-Brazil was 67.16%, which led to more than one item for each of the identified factors.
The BHQ-Brazil presented a moderate convergent validity with the mMRC scale, demonstrating that the higher the dyspnea, the lower the BHQ score, determining a worse quality of life. This was probably because the mMRC scale assesses dyspnea during activities of daily living, whereas the BHQ addresses other symptoms related to bronchiectasis. Spinou et al.20 observed a strong correlation between the BHQ and dyspnea assessed using the visual analog scale; however, this scale evaluates shortness of breath only and does not consider dyspnea during activities of daily living.
The BHQ-Brazil was able to discriminate the impact of the quality of life on those with the highest number of exacerbations, colonization by Pseudomonas aeruginosa, worse dyspnea scale scores, and greater number of affected lung lobes. These clinical markers are related to the phenotypes of individuals with an exacerbating profile.6 The exacerbation and pulmonary colonization by Pseudomonas aeruginosa in individuals with bronchiectasis are considered important markers of reduced quality of life.3,5,6,38 The BHQ-Brazil exhibited strong concurrent validity with the SGRQ with an advantage of a fast application and easy interpretation. The rapid application was confirmed by the shorter time required to complete the questionnaire (3.8...min) compared with both the SGRQ (12...min)11...14 and QoL-B (10...min).8...10
The BHQ-Brazil showed a small SEM and a narrow SDC, indicating a very good agreement with little response variability between test-retest. These psychometric properties had not been evaluated since the publication of the original, Korean, and Danish BHQ versions.20...22 Also, no ceiling and floor effects were observed, as none of the participants obtained the lowest or highest scores in the BHQ-Brazil. Thus, the BHQ-Brazil can be used to assess the response to interventions such as pulmonary rehabilitation or pharmacological treatments. Besides, BHQ scores may also differ during and after exacerbations. Nevertheless, prospective studies must be conducted to confirm these assumptions.
This study had some limitations. Individuals were recruited from a single referral center for bronchiectasis in S.·o Paulo, which receives patients from all over Brazil. Thus, it is essential to administer the BHQ-Brazil in other states of the country to test its external validity. Moreover, the study included young individuals with less severe bronchiectasis. Thus, future studies focusing on assessing the usefulness of BHQ in an older population and people with different disease severities are suggested.
In conclusion, the Brazilian-Portuguese version of the BHQ presents adequate measurement properties to evaluate the quality of life of individuals with bronchiectasis and can be implemented in clinical practice.
Ethical approvalAll procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Moreover, informed consent was obtained from all individuals included in the study (certification number: 2.532.903 and 2.574.759).
Source of financial supportAL was supported by Coordena...·o de Aperfei..oamento de Pessoal de N.ível Superior (CAPES, number 1823466). COC was supported by CAPES (number 1803009). SDC is suported by Conselho Nacional de Desenvolvimento Cient.ífico e Tecnol..gico (number 306531/2018-6).
Conflicts of interestThe authors have no conflicts of interest to declare.
Contributions of each author to the paperAL: Performed the data collection, conceived and designed the study, analyzed the data, read and approved the manuscript.
COC: Performed the data collection, conceived and designed the study, analyzed the data, read and approved the manuscript.
SSB: Conceived and designed the study, read and approved the manuscript.
ACL: Analyzed the data, read and approved the manuscript.
SZR: Conceived and designed the study, read and approved the manuscript.
RAA: Conceived and designed the study, read and approved the manuscript.
RE: Conceived and designed the study, read and approved the manuscript.
SDC: Conceived and designed the study, analyzed the data, read and approved the manuscript and is the guarantor of the study.