Accelerated subcutaneous immunotherapy (SCIT) schedules represent an alternative to conventional SCIT, providing immunotherapy benefits in a shorter period of time. The objectives of this systematic review were to assess clinical and immunological efficacy as well as safety of accelerated SCIT build-up schedules for the treatment of respiratory allergy in pediatric patients.
MethodsStudies were located by searching PubMed, using “immunotherapy” and “desensitization” as keywords. The selection of studies, published from January 1st, 2006, to December 31th, 2015, was performed in two stages: screening of titles and abstracts, and assessment of the full papers identified as relevant, considering the inclusion criteria. Data were extracted in a standardized way and synthesized qualitatively to assess efficacy and safety of accelerated schedules in respiratory allergy.
ResultsEleven trials were included: two evaluated rush SCIT and nine assessed cluster SCIT. This review demonstrated that rush and cluster schedules are clinically and immunological efficacious, with faster effect than conventional schedules. No relevant difference with respect to clinical outcomes was noticed between subgroups (pediatric, adult and mixed populations). Regarding safety, most local adverse reactions were mild and there were neither life-threatening systemic reactions nor fatal events. No relevant differences in the incidence and severity of either local or systemic reactions between the accelerated schedule group and control group were registered.
ConclusionsAccelerated SCIT build-up schedules are effective in the treatment of respiratory allergy in pediatric patients, representing a safe alternative to the conventional schedules with the advantage of achieving clinical effectiveness sooner.
Currently three therapeutic approaches are employed for IgE-mediated respiratory allergies treatment: specific allergen avoidance, symptomatic drugs such as antihistamines, corticosteroids, mast cell stabilizers, antileukotrienes, β2-agonists and anti-IgE monoclonal antibodies, and allergen-specific immunotherapy (SIT). SIT is an immune-modifying therapeutic since it restores mechanisms of immune tolerance to allergens, resulting in a significant reduction of symptoms and symptomatic medication usage, as well as in an improvement of quality of life and productivity at school and/or work.1–4 It is of particular interest in pediatric population because of its capacity to change the response to allergens at an early phase and, thus, to prevent disease progression.5
Subcutaneous immunotherapy (SCIT) protocols are performed in two stages: build-up (up-dosing) phase which involves the administration of increasing doses of allergen extracts until the effective (or maintenance) dose is reached, and maintenance phase. Conventional immunotherapy schedules generally involve one or two weekly injections during up-dosing phase, over a 16-week period, followed by monthly maintenance injections for a period of three to five years. Rush and cluster immunotherapy schedules are accelerated build-up schedules which allow the patient to reach the maintenance dose and, thus, the benefits of immunotherapy, more rapidly. In a cluster up-dosing regimen, two to four repeated injections are given to the patient in a single day of treatment on nonconsecutive days, in most cases reaching the maintenance dose in four to eight weeks. A rush up-dosing schedule involves the subcutaneous administration of increasing amounts of allergen extracts at intervals of 15–60min over a period ranging from one to three days.4,6
It is estimated that only a few allergic patients accept this therapeutic option mainly because of time inconvenience. Thus, accelerated schedules represent an alternative to conventional time-consuming schedules, allowing a reduced number of office visits (and associated costs), while preserving clinical efficacy. Despite their advantages, these schedules have not been widely used, mainly due to safety issues.6
The main objectives of this systematic review were to evaluate clinical and immunological efficacy as well as safety of accelerated SCIT build-up schedules for the treatment of respiratory allergy in pediatric patients.
MethodsThe protocol was developed following international guidelines for systematic reviews.7
Studies were obtained by searching PubMed, from January 1st, 2006, to December 31st, 2015. The search strategy used two keywords: “immunotherapy” and “desensitization”. Inclusion criteria used to select studies were: (i) population: studies of participants diagnosed with IgE-mediated allergic respiratory disease, confirmed by objective measures (positive skin prick test and/or serum-specific IgE to sensitizing allergens); (ii) intervention: rush or cluster SCIT; (iii) comparative intervention: placebo, conventional SCIT or pharmacotherapy; (iv) outcomes: symptoms and medication scores, quality of life, functional measures (lung function, rhinometry), allergen specific reactivity (cutaneous, nasal, conjunctival, and bronchial allergen reactivity), immunological and inflammatory parameters, safety; and (v) study design: randomized controlled trial (RCT). Only studies written in English were included.
The first stage of studies selection was a screening of titles and abstracts against the inclusion criteria to identify potentially relevant articles. When a definite decision based on title or abstract was not possible, the full papers were assessed. Rejected studies were grouped into those that did not meet the review objectives and those that addressed the topic of interest but failed on one or more inclusion criteria. Studies were also excluded when there was no abstract available. The second stage was the assessment of the full papers identified as relevant at time of the initial screening. If there were no full papers to access, those studies were excluded.
Only essential information for descriptive purposes of the systematic review were included in data extraction forms, namely: first author; publication year; study design; subjects characteristics (age, disease and co-morbidities) and number of subjects allocated to intervention and control groups; intervention description (type of vaccine, build-up schedule, duration and number of injections per up-dosing visit, gap between increasing doses) and control group; co-interventions description; treatment duration; outcome measures; and key results of the study analysis. The Cochrane Collaboration's recommended tool for assessing risk of bias7 was the quality assessment process used in this review.
Data were stratified according to subject age (18 years or under – pediatric population; over 18 years – adult population; or mixed population – pediatric and adult populations), up-dosing schedule (rush or cluster) and outcomes, and were synthesized qualitatively. Clinical efficacy was evaluated by means of the following outcomes: symptoms and medication scores, quality of life assessment, functional measures and allergen specific reactivity. Immunological efficacy was determined according to objective parameters: serum antibodies analysis, lymphocyte subsets and cytokines, and inflammatory markers. Regarding safety, adverse reactions were analyzed according to location (local or systemic), and compared between groups concerning severity, time of appearance (immediate or delayed), requirement of symptomatic treatment, dose adjustments or withdrawals, and phase of SCIT protocol (induction or maintenance).
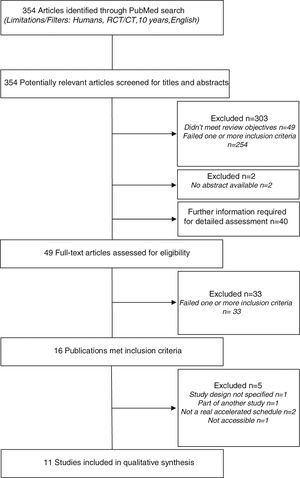
ResultsStudy identification and selectionA preliminary database search identified a total of 354 potentially relevant articles. Of these, 303 studies were excluded: 49 did not meet the review objectives and 254 failed on one or more inclusion criteria. Two studies were excluded because abstracts were not available.
A definite decision based on title or abstract was not possible in 40 cases, hence the full papers were obtained to evaluate if the inclusion criteria were or were not satisfied. In addition nine other potentially relevant articles (not excluded based on title and abstract) were assessed for eligibility. In total, 49 full-text articles were retrieved and assessed for eligibility. Of them, 33 were excluded because they failed on one or more criteria.
The remaining 16 publications met all inclusion criteria. However, five of these studies were excluded: one did not elucidate the study design, one was part of another study, two had no real accelerated build-up schedule (rush or cluster SCIT), and one was inaccessible.
In the end, 11 studies were included in the systematic review. The flowchart of studies selection is shown in Fig. 1.
Study and population characteristicsOverall characteristics of included studies and subjects are listed in Table 1.
Characteristics of studies included in the systematic review.
| Study (year) | Study design | Study's group (N) | Age (mean±SD) | Disease (co-morbidities) | Intervention/Comparator | Vaccine type | Build-up schedule (duration) | No. injections/visit (gap between doses) | Total duration | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|
| Klimek et al. (2014)12 | RCT (DBPC) | I (N=61) | 37.1±10.4yrs | Rhinitis/Rhinoconjunctivitis (with or without asthma) | Grass Rye | Allergoid | Cluster (1wk) | 2/2 (30min) | 1yr | SMS/SS/MS NCT Immune parameters Safety |
| C (N=59) | 36.2±10.7yrs | Placebo | – | Cluster (1wk) | 2/2 (30min) | |||||
| Pfaar et al. (2013)15 | RCT (DBPC) | I (N=186) | 31.3±12.4yrs 15.3±1.9yrsa | Rhinitis/Rhinoconjunctivitis (with or without asthma) | Birch Grass | Allergoid | Rush (1 day) | 2 (30min) | 2yrs | SMS/SS/MS QoL Immune parameters Safety |
| C (N=99) | 31.3±11.8yrs 15.3±1.8yrsa | Placebo | – | Rush (1 day) | 2 (30min) | |||||
| Lou et al. (2012)8 | RCT (OPS) | I (N=25) | 12yrs | Rhinitis/Rhinoconjunctivitis (without asthma) | Der p | Depot | Cluster (6wk) | 3/2/2/2/2/2/1 (1h) | 1yr | SS/MS Immune parameters |
| C (N=25) | 11yrs | Pharmacotherapy | – | – | – | |||||
| Pfaar et al. (2012)16 | RCT (DBPC) | I (N=135) | 32.9±13.8yrs | Rhinitis/Rhinoconjunctivitis (with or without asthma) | Grass | Allergoid | Rush (1 day) | 2 (30min) | 2yrs | SMS/SS/MS QoL Immune parameters Safety |
| C (N=60) | 33.8±13.3yrs | Placebo | – | Rush (1 day) | 2 (30min) | |||||
| Vidal et al. (2011)17 | RCT (DBPC) | I (N=21) | 25.9yrs | Asthma (with or without rhinoconjunctivitis) | Der p | Depot | Cluster (3wks) | 2/2/2/2 (30min) | 1yr 5mon | ST Immune parameters Safety |
| C (N=24) | 28.3yrs | Placebo | – | Cluster (3wks) | 2/2/2/2 (30min) | |||||
| Schubert et al. (2008)9 | RCT (OPS) | I (N=22) | 10yrs | Asthma | Der p | Depot | Cluster (5wks) | 3/3/3/2/1/1 (NAD) | 16wks | Immune parameters Safety |
| C (N=12) | 8.5yrs | Der p | Depot | Conventional (13wks) | 1/1/1/1/1/1/1/1/1/1/1/1/1/1 (1wk) | |||||
| Zhang et al. (2008)18 | RCT (OPS) | I (N=48) | 25yrs | Rhinitis/Rhinoconjunctivitis (with or without asthma) | Der p | Depot | Cluster (6wks) | 3/2/2/2/2/2/1 (30min) | 1yr | SS/MS QoL ST Immune parameters Safety |
| C (N=48) | 25yrs | Der p | Depot | Conventional (14wks) | 1/1/1/1/1/1/1/1/1/1/1/1/1/1/1 (1wk) | |||||
| Blumberga et al. (2006)13 | RCT (DBPC) | I (N=26) | 29.8±10.7yrs | Asthma | Der p | Depot | Cluster (8wks) | 2–3/visit (NAD) | 3yrs | SS/MS Lung function Safety |
| C (N=28) | 28.5±7.1yrs | Placebo | – | Cluster (8wks) | 2–3/visit (NAD) | |||||
| Colás et al. (2006)14 | RCT (DBPC) | I (N=43) | 34yrs | Rhinitis/Rhinoconjunctivitis (with or without asthma) | Sal k | Allergoid | Cluster (1wk) | 3/3 (15–20min) | 1yr 1wk | SS/MS QoL ST Safety |
| C (N=20) | 33yrs | Placebo | – | Cluster (1wk) | 3/3 (15–20min) | |||||
| Roberts et al. (2006)10 | RCT (DBPC) | I (N=19) | 9.2±4.4yrs | Asthma (with rhinoconjunctivitis) | Grass | Depot | Cluster (11wks) | 3/2/2/2/2/2/1/1 (30–60min) | 1yr 2mon | SMS/SS/MS Lung function ST/CCT/BCT Immune parameters Safety |
| C (N=18) | 10.6±2.9yrs | Placebo | – | Cluster (11wks) | 3/2/2/2/2/2/1/1 (30–60min) | |||||
| Ibero et al. (2006)11 | RCT (OPS) | I (N=15) | 10yrs | Asthma (with rhinoconjunctivitis) | Der p | Allergoid | Cluster (1wk) | 2/2 (30min) | 4mon 1wk | SS/MS ST/BCT Safety |
| C (N=15) | 12yrs | Pharmacotherapy | – | – | – |
N, number of participants; SD, standard deviation; RCT, randomized controlled trial; DBPC, double-blind placebo-controlled trial; OPS, open-label and parallel study; I, intervention group; C, control group; wk, week; yr, year; mon, month; SMS, combined symptoms-medication score; SS, symptoms score; MS, medication score; QoL, quality of life; ST, Skin test; NCT, nasal challenge test; CCT, conjunctival challenge test; BCT, bronchial challenge test; Der p, Dermatophagoides pteronyssinus; Sal k, Salsola kali; NAD, no available data.
Four studies8–11 integrated participants at age of 18 years or under, three12–14 included adult participants, and the remaining four15–18 were of mixed population.
Two studies15,16 described a rush induction schedule. In both studies each individual received two injections at up-dosing visit, with a 30min gap and a one day build-up phase.
A cluster schedule was described in the remaining nine studies.8,17,18 During the build-up phase, the number of injections ranged from four to 15, with a 15–60min gap between doses. Time required for induction phase ranged from one to 11 weeks. A great heterogeneity in the allergen dose delivered and the reported pharmacological units was observed.
Risk of bias assessmentThe method used to generate random sequence and to conceal allocation sequence was adequately performed in four studies10,12,15,18 (“low risk” of selection bias). In the remaining seven studies8,9,11,13,14,16,17 the methods were poorly reported making it difficult to evaluate (“unclear risk”).
Blinding of participants and investigators was clearly stated and not broken in seven studies10,12–17 (“low risk” of performance bias). Four8,9,11,18 studies were not blinded (“high risk”).
Three studies9,11,18 did not conduct a blinding assessment of patient-reported outcomes (symptoms and medication scores, quality of life assessment, and safety evaluation) and had a “high risk” of detection bias, while eight studies8,10,12–17 described all measures used to blind outcome assessors (“low risk”). All included studies8–18 were classified with “low risk” of detection bias regarding objective outcomes such as functional measures, allergen specific reactivity, immunological and inflammatory parameters.
In all included studies8–18 missing data was imputed using appropriate methods such as intention-to-treat (ITT) analysis or, if an ITT analysis was not performed, the numbers and reasons for withdrawals or exclusions from the study were reported (“low risk” of attrition bias).
Cluster SCIT in pediatric patientsThree studies8,10,11 evaluated clinical efficacy of cluster SCIT in pediatric patients using symptoms and/or medication scores. Roberts et al.10 showed a significant reduction in asthma symptom and medication score (SMS) in the active group compared to placebo, and demonstrated an improvement in individual symptom score (SS) and medication score (MS) in the cluster SCIT group but the differences between study groups were not statistically significant. Lou et al.8 reported that rhinoconjunctivitis total SS was significantly decreased from baseline for both SCIT-treated and drug-treated patients, however without significant differences between groups. In contrast, the MS was significantly reduced from baseline and the differences between groups were statistically significant. Ibero et al.11 showed a significant reduction in the total SS of the active group against the control group. The MS was reduced from baseline for both groups but without values reaching statistical significance.
Data on functional measures were available in one trial10. Roberts et al.10 did not demonstrate a significant effect of immunotherapy on lung function of asthmatic children using the parameter Forced Expiratory Volume at one second (FEV1).
Two studies10,11 assessed clinical efficacy by skin prick tests, and conjunctival and bronchial challenge tests. Roberts et al.10 and Ibero et al.11 showed a statistically significant increase in the concentration of allergen extract needed to produce positive cutaneous, conjunctival and bronchial reactions in the active group compared to placebo, with significant differences between groups.
Two studies8,9 assessed immunogenicity of cluster SCIT in pediatric patients. Schubert et al.9 quantified Der p-specific IgE, IgG and IgG4 levels at week one, eight and 16 of treatment. Specific IgE concentrations increased significantly at week eight (end of the rapid build-up phase) in the cluster group. There was no significant increase in IgE levels conventional SCIT group. Specific IgG and IgG4 showed a significant increase at week eight in the cluster group, and at week 16 (end of the classic build-up phase) in the control group. Schubert et al.9 also evaluated inhibitory capacities of serum IgG antibodies induced by specific immunotherapy on the allergen-induced cysteinyl leukotrienes (cysLT) release and CD63 expression on basophils. CysLT secretion significantly decreased after eight weeks in the cluster group, and after 16 weeks in the conventional SCIT group. CD63 expression showed a significant reduction in both groups after eight weeks, but in the conventional group it continued to decrease at week 16 and in the cluster group it reached a plateau level at week eight. Lou et al.8 demonstrated that the changes in IgE levels from baseline were not statistically significant in both groups but the concentration of allergen-specific IgG4 showed a significant increase from baseline in actively-treated patients compared with those in the control treatment group, after one year of immunotherapy.
Two trials8,9 analyzed T-cell subsets and cytokines secretion. Schubert et al.9 evaluated the effect of cluster and conventional SCIT on the balance of Treg, Th1 and Th2 cells transcription factors (Foxp3, T-bet and GATA-3, respectively) and did not find significant differences within and between treatment groups. Lou et al.8 found that the frequencies of Th1 and Th2 cells were not significantly changed from baseline in active and control groups but demonstrated a significant increase in Tr1 cells in SCIT-treated patients. Significant correlations were found between increased numbers of Tr1 cells and improvements in clinical severity, particularly in nasal symptoms, in SCIT group. Lou et al.8 also demonstrated that the levels of IL-10 were significantly increased in the active treatment group, but not in the control group, after one year, as opposed to the levels of IFN-γ and IL-4 which did not change in both groups.
Two clinical trials9,10 evaluated airway inflammatory markers. Roberts et al.10 found that there were no significant differences in the levels of exhaled nitric oxide (eNO) and number of eosinophils per gram of sputum between the actively-treated and placebo subjects. Schubert et al.9 reported that there was a decrease of eNO levels in the cluster group over the treatment period, without significant differences when compared with that seen in the conventional SCIT group, while both groups showed a reduction of eosinophilic cationic protein (ECP) levels compared with baseline values, but the cluster group had a more rapid decline of ECP and the conventional SCIT group reached a significant decline only after 16 weeks.
Three studies9–11 evaluated safety of cluster SCIT in pediatric population summarized in Table 2. Roberts et al.10 reported a total of 54 treatment-related adverse reactions. Patients in the intervention group experienced 34 reactions, of which 13 were local and 21 were systemic. The remaining 20 reactions occurred in the placebo group: 11 local reactions and nine systemic reactions. All local and systemic reactions were mild and well tolerated with specific treatment. Four pulmonary events occurred during up-dosing phase and three during maintenance phase. In this trial pretreatment with topical anesthetic cream and an antihistamine was administered before immunotherapy injections. Schubert et al.9 reported 185 local adverse effects in the cluster group which corresponds to 54.2% of a total of 341 cluster injections, and 80 local adverse reactions occurred among patients in the conventional SCIT group which corresponds to 53% of a total of 151 classic injections. Subjects of the cluster group experienced 12 (3.5% of cluster injections) systemic adverse reactions. In the conventional SCIT group, the investigators reported seven (4.6% of classic injections) systemic adverse events. There were no significant differences between the two treatment groups regarding local and systemic adverse reactions. None subject dropped out because of adverse events however an increase of systemic side effects, mainly cough, led to five patients in the cluster group to receive a lower dose of vaccine at week four of treatment. Ibero et al.11 described three local reactions in three patients and two systemic reactions in two patients in the active group. One child experienced pain and heat during up-dosing phase. The other two local reactions and all systemic reactions occurred during maintenance phase.
Safety evaluation of accelerated SCIT build-up schedules in pediatric population.
| Subgroup | Study | Intervention/comparator | Adverse reactions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of reactions | Frequency of reactions (r/%r) | Description of reactions (r/%r) | Severity of reactions (r/%r) | Specific treatment/dose adjustment (yes/no) | Study withdrawals (n) | Protocol phase (r) | |||||||||
| Cluster SCIT | Roberts et al. (2006)10 | Cluster vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | |
| Local | 13 | 11 | Pruritus, Pain, Swelling (13) | Pruritus, Pain, Swelling (11) | Mild (13) | Mild (11) | Yes | Yes | 0 | 0 | NAD | NAD | |||
| Systemic | 21 | 9 | Eczema, Urticaria, RCA (most) Cough (1) Pulmonary (4) | Eczema, Urticaria, RCA (most) Chest tightness (1) Pulmonary (3) | Mild (21) | Mild (9) | Yes | Yes | 0 | 0 | Up-dosing (4) | Maintenance (3) | |||
| Schubert et al. (2008)9 | Cluster vs. conventional | I | C | I | C | I | C | I | C | I | C | I | C | ||
| Local | 185 54.2% | 80 53% | Erythema/Redness (97/28.4%) Swelling <5cm (57/16.7%) Swelling >5cm (22/6.5%) Painful swelling >3h (8/2.3%) | Erythema/Redness (40/26.5%) Swelling <5cm (20/13.2%) Swelling >5cm (17/11.3%) Painful swelling >3h (3/2%) | Mild (185) | Mild (80) | No | No | 0 | 0 | NAD | NAD | |||
| Systemic | 12 3.5% | 7 4.6% | Cough (10/2.9%) Dyspnea (2/0.6%) | Cough (6/4%) Dyspnea (1/0.7%) | Gr I-II (2.9%) Gr III (0.6%) | Gr I-II (3.9%) Gr III (0.7%) | Yes | No | 0 | 0 | NAD | NAD | |||
| Ibero et al. (2006)11 | Cluster vs. pharmacotherapy | I | C | I | C | I | C | I | C | I | C | I | C | ||
| Local | 3 | NAD | Pain (1) Pain and heat (1) Induration and pruritus (1) | NAD | Mild (3) | NAD | No | NAD | 0 | 0 | Up-dosing (1) Maintenance (2) | NAD | |||
| Systemic | 2 | NAD | Dyspnea (1) Asthma and rhinitis (1) | NAD | Gr II (2) | NAD | No | NAD | 0 | 0 | Maintenance (2) | NAD | |||
I, intervention group; C, control group; r, number of adverse reactions; %r, percentage of injections with adverse reactions; n, number of subjects; RCA, rhinoconjunctivitis; Gr, grade; NAD, no available data.
Three studies12–14 assessed clinical efficacy of cluster SCIT in adults by measuring changes in symptoms severity and medication usage. Klimek et al.12 found a significant reduction in rhinoconjunctivitis SMS, SS and MS in the actively-treated group compared to placebo. Blumberga et al.13 demonstrated that there were no largely changes at the annual re-assessments in SS and MS. Colás et al.14 reported significant reduction in total rhinoconjunctivitis SS and MS in the active group compared to placebo.
One clinical trial14 reported the Quality of Life Questionnaire (QLQ) as a secondary outcome for cluster SCIT clinical efficacy. Colás et al.14 demonstrated that there was a greater improvement in overall quality of life (QOL) of patients in the active group, but not in the placebo group, with significant differences between groups.
One clinical trial13 used lung function to assess clinical efficacy. Blumberga et al.13 reported that peak expiratory flow (PEF) did not show significant changes overall the treatment period.
Two studies12,14 quantified allergen specific reactivity through skin tests and nasal challenge test. Klimek et al.12 showed an increase in allergen concentration required to induce a positive nasal reaction in both active and placebo groups, however the differences between groups were not statistically significant. Colás et al.14 demonstrated that the allergen concentration needed to produce a positive cutaneous reaction was significantly higher in the active group compared to placebo.
One clinical trial12 analyzed allergen-specific IgE and IgG4 levels as immunological efficacy markers of cluster SCIT in adults. Klimek et al.12 described a significantly higher increase of IgG4 levels in the active group than in the control group with significant differences between groups, while the concentration of IgE was decreased in both groups without statistically significant differences between groups.
All adult studies12–14 evaluated the safety of cluster SCIT summarized in Table 3. Klimek et al.12 reported that local reactions occurred after 0.7% of the total of 928 injections given to the subjects in the active group. All of them were grade ≥I on the European Academy of Allergy and Clinical Immunology (EAACI) scale and occurred during the up-dosing phase. Two mild systemic events (rhinitis and nasal obstruction) also occurred during the up-dosing phase, after 0.2% of the total of 928 injections, and were classified as immediate reactions. Delayed grade I systemic reactions (fatigue, nasal obstruction, skin reaction) were reported after 0.6% of allergoid injections. Blumberga et al.13 reported 43 systemic reactions which correspond to a rate of 4.7% systemic reactions per cluster injection. On the other hand, there were a total of 21 systemic adverse events in the placebo group with a rate of 2.1% of systemic reactions per placebo injection. Most systemic adverse events occurred during up-dosing phase in both groups. There were no withdrawals from the study due to adverse reactions, but one patient in the intervention group experienced a severe bronchospasm which required specific treatment. Colás et al.14 reported 16 local adverse reactions in SCIT-treated patients and 10 local side effects in placebo subjects. All local reactions were delayed, including nine and eight subcutaneous nodules caused by aluminum hydroxide in the intervention and placebo groups, respectively. The remaining local events were clinically irrelevant (diameter of less than 10cm). There were 16 systemic adverse reactions in the active group vs. four in the placebo group.
Safety evaluation of accelerated SCIT build-up schedules in adult population.
| Subgroup | Study | Intervention/comparator | Adverse reactions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of reactions | Frequency of reactions (r/%r) | Description of reactions (r/%r) | Severity of reactions (r/%r) | Specific treatment/Dose adjustment (yes/no) | Study withdrawals (n) | Protocol phase (r/%r) | |||||||||
| Cluster SCIT | Klimek et al. (2014)12 | Cluster vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | |
| Local | 0.7% | NAD | Immediatea (0.7%) | NAD | Gr ≥I (0.7%) | NAD | NAD | NAD | 0 | 0 | Up-dosing (0.7%) | NAD | |||
| Systemic | 0.8% | NAD | Immediatea (0.2%): rhinitis, nasal obstruction Delayedb (0.6%): fatigue, nasal obstruction, skin reaction | NAD | Gr I (0.8%) | NAD | NAD | NAD | 0 | 0 | Up-dosing (0.2%) | NAD | |||
| Blumberga et al. (2006)13 | Cluster vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | ||
| Local | NAD | NAD | NAD | NAD | NAD | NAD | NAD | NAD | NAD | NAD | NAD | NAD | |||
| Systemic | 43 | 21 | NAD | NAD | Gr II (41) Gr III (2) | Gr I (9) Gr II (12) | Yes | NAD | 0 | 0 | Up-dosing (most) | Up-dosing (most) | |||
| Colás et al. (2006)14 | Cluster vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | ||
| Local | 16 | 10 | Delayedb (16): subcutaneous nodules (9) | Delayedb (10): subcutaneous nodules (8) | Irrelevant (d<10cm) | Irrelevant (d<10cm) | No | No | 0 | 0 | NAD | NAD | |||
| Systemic | 16 | 4 | Immediatea (4) Delayedb (12): RCA, otic pruritus | Delayedb (4): RCA, otic pruritus | Gr II (16) | Gr II (4) | No | No | 0 | 0 | NAD | NAD | |||
I, intervention group; C, control group; r, number of adverse reactions; %r, percentage of injections with adverse reactions; n, number of subjects; RCA, rhinoconjunctivitis; Gr, grade; d, diameter of local reaction; NAD, no available data.
Immediate reaction=onset within the first 30min after injection.
Delayed reaction=onset after 30min post-vaccine.
Two placebo-controlled trials15,16 evaluated the clinical efficacy of rush SCIT in mixed population using the combined SMS as primary efficacy endpoint. One study15 found a significant reduction in rhinoconjunctivitis SMS in the active group but the individual SS and MS were not significantly different between groups. Another study16 reported a significant reduction in rhinoconjunctivitis SMS, SS and MS for the active group compared to placebo.
Two clinical trials15,16 reported on QLQ as a secondary outcome and demonstrated a significant reduction in total QOL score for the active group compared to placebo.
Two clinical trials15,16 determined allergen-specific IgE and IgG4 levels and reported a significantly higher increase of IgG4 concentration in the active group than in the placebo group. One of these studies15 showed that there was no significant correlation between either levels of IgG4 at the end of the treatment or increase in IgG4 from baseline and the combined SMS. No significant changes in IgE levels were observed throughout the treatment in both trials.
Two studies15,16 assessed safety of rush SCIT in mixed population summarized in Table 4. One study15 observed local side effects in 66 of 186 (35.5%) actively-treated patients and in 29 of 99 (29.3%) placebo subjects. None of the local adverse reactions were serious. Thirteen systemic reactions were observed in 10 patients treated with rush SCIT; of these, two asthmatic reactions were grade II on the EAACI scale, and the remaining reactions were grade I. Five systemic adverse events occurred in four placebo patients, and all were grade I. The other study16 reported local reactions in 95 of the 135 (70.4%) subjects from the active group and in 24 of the 60 (40%) placebo subjects. Twenty-seven systemic reactions occurred in 16 patients treated with rush SCIT and seven appeared in three placebo subjects. In total, four patients from the intervention group and two patients from the control group withdrew from the study because of adverse reactions.
Safety evaluation of accelerated SCIT build-up schedules in mixed population.
| Subgroup | Study | Intervention/comparator | Adverse reactions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of reactions | Frequency of reactions (r/%r) | Description of reactions (r/%r) | Severity of reactions (r/%r) | Specific treatment/Dose adjustment (yes/no) | Study withdrawals (n) | Protocol phase (r) | |||||||||
| Rush SCIT | Pfaar et al. (2013)15 | Rush vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | |
| Local | 66/186 patients | 29/99 patients | NAD | NAD | No serious | No serious | No | No | 0 | 0 | NAD | NAD | |||
| Systemic | 13 | 5 | Vertigo/anxiety (2) Asthma (2) Conjunctivitis (2) Rhinitis (1) Throat irritation (1) Headache (1) Chills (2) Pruritus (1) Feeling hot (1) | NAD | Gr I (11) Gr II (2) | Gr I (5) | No | No | 0 | 0 | NAD | NAD | |||
| Pfaar et al. (2012)16 | Rush vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | ||
| Local | 95/135 patients | 24/60 patients | NAD | NAD | NAD | NAD | No | No | 4 | 2 | NAD | NAD | |||
| Systemic | 27 | 7 | NAD | NAD | Gr I-II (34) | NAD | NAD | NAD | NAD | ||||||
| Cluster SCIT | Vidal et al. (2011)17 | Cluster vs. placebo | I | C | I | C | I | C | I | C | I | C | I | C | |
| Local | 10 | 4 | NAD | NAD | NAD | NAD | NAD | NAD | 1 | 0 | NAD | NAD | |||
| Systemic | 8 | 6 | NAD | NAD | Mild (5) Moderate (3) | Mild (3) Moderate (3) | NAD | NAD | 0 | NAD | NAD | ||||
| Zhang et al. (2008)18 | Cluster vs. conventional | I | C | I | C | I | C | I | C | I | C | I | C | ||
| Local | 18 3.4% | 14 3% | Immediatea | Immediatea | Irrelevant (d<5cm) | Irrelevant (d<5cm) | No | No | 0 | 0 | Up-dosing (11) Maintenance (7) | Up-dosing (9) Maintenance (5) | |||
| Systemic | 11 2.5% | 10 2.2% | Immediatea | Immediatea | Gr I (7) Gr II (4) | Gr I (7) Gr II (3) | Yes | Yes | 0 | 0 | Up-dosing (5) Maintenance (6) | Up-dosing (6) Maintenance(4) | |||
I, intervention group; C, control group; r, number of adverse reactions; %r, percentage of injections with adverse reactions; n, number of subjects; Gr, grade; NAD, no available data.
Immediate reaction=onset within the first 30min after injection.
One study18 evaluated clinical efficacy of cluster SCIT in a mixed population through symptoms and medication scores. Zhang et al.18 determined clinical efficacy of cluster SCIT for patients with allergic rhinoconjunctivitis at week zero (baseline), six (end of the cluster up-dosing phase), 14 (end of the conventional up-dosing phase) and 52 (end of the treatment). At week six, the cluster group showed a significant decrease in SS and MS compared with baseline values, as opposed to that seen in the conventional group. At week 14, both groups showed a significant decrease in both scores compared with baseline, but while the reduction in SS was significantly greater in the cluster group, the differences between groups had disappeared in respect to MS. At the end of the study, the differences between groups were minimal in both scores.
One clinical trial18 reported the QLQ as a secondary outcome for cluster SCIT clinical efficacy. Zhang et al.18 showed a significant improvement in overall QOL in both groups, with no significant differences between them.
Two studies17,18 quantified allergen specific reactivity through skin tests. Vidal et al.17 observed that the Cutaneous Tolerance Index was significantly reduced in the active group as opposed to the placebo group, so there were statistically significant differences between groups. Zhang et al.18 showed that both cluster and conventional SCIT decreased the Cutaneous Tolerance Index, but there were no significant differences between groups.
Two trials17,18 reported a significant increase in specific IgG4 levels in the active, but not in the placebo group, with significant differences between groups. There were no relevant differences of levels of specific IgE between groups. Vidal et al.17 also evaluated the inhibitory capacity of non-IgE antibodies on IgE binding to allergens and demonstrated a significantly higher inhibitory effect in the active group, not found in the placebo group. Zhang et al.18 reported that IgE levels did not significantly change after one year of treatment in either group and there were no significant differences between cluster and conventional schedules.
Two studies17,18 evaluated safety of cluster SCIT in mixed population summarized in Table 4. Vidal et al.17 found 14 local adverse events: 10 of these events occurred in the intervention group and four adverse reactions were observed in placebo subjects. The investigators observed 14 systemic reactions: eight of them occurred in patients treated with SCIT, and the remaining six reactions occurred in the placebo group. Interestingly among the total systemic reactions observed, only two were considered as treatment-related, one in each study group. Only one withdrawal was reported in the intervention group due to adverse events. Zhang et al.18 reported 11 local adverse reactions in the cluster group during up-dosing phase, so local adverse reactions occurred in 1.7% of all up-dosing injections. On the other hand, nine local adverse events occurred in the conventional SCIT group during up-dosing phase, so local reactions were triggered by 1.4% of all classic injections. During maintenance phase, seven local adverse reactions were observed, this is, 1.7% of all maintenance injections in the cluster group, and five reactions, which corresponds to 1.6% of all maintenance injections, in the conventional group. All local reactions were clinically irrelevant (diameter lesser than five centimeters) and there were no differences in frequency and severity of local reactions between groups. In the intervention group there were 11 systemic reactions, five during up-dosing phase (1% of all up-dosing injections) and six during maintenance phase (1.5% of all maintenance injections). In the conventional SCIT group six systemic reactions during up-dosing phase (0.9% of all up-dosing injections) occurred. During maintenance phase four systemic reactions were reported. There were no differences in frequency and type of systemic reactions between groups. All systemic reactions were successfully treated. It should be noted that premedication (antihistamine) was used before each immunotherapy injection.
DiscussionThe present systematic review was carried out to assess clinical and immunological efficacy and safety of accelerated SCIT schedules in respiratory allergy.
This systematic review demonstrated that rush and cluster SCIT were clinically efficacious and had more rapid effects compared with a conventional schedule. No relevant differences between subgroups (pediatric, adult and mixed populations) were observed for cluster SCIT. Although rush SCIT was only evaluated in mixed population trials, the studies noted that pediatric patients showed similar results to those of adults. However, careful attention must be paid in the interpretation of these data since there was a significant heterogeneity between studies, mainly related to the variety of methods and scoring systems used to determine clinical efficacy of immunotherapy.
Regarding immunological efficacy, this systematic review showed that allergen-specific IgG4 significantly increased in the intervention group compared to the control group, reflecting the immunogenicity of the treatment, which was more rapidly achieved in the accelerated than in the classic schedules. Since changes in IgE levels were not constant among studies, they are not as consistent as IgG4 levels as indicators of successful immunotherapy. Additionally, blocking activity of IgG antibodies was an important finding that supports immunological efficacy of accelerated immunotherapy schedules. This review also showed allergen-specific Treg cells and IL-10 as important markers of effective desensitization. Nevertheless, clear correlations between immunological parameters and clinical outcomes are scarce, and in this review only one study8 found significant correlations between increased numbers of Treg cells and improvement in clinical severity.
The main obstacle to the widespread implementation of accelerated schedules is the potential risk of side effects, particularly in children. However, this descriptive analysis did not reveal relevant differences in the incidence of either local or systemic adverse reactions between the accelerated schedules and controls, demonstrating a good safety profile for these regimens in children and adults. Overall local adverse reactions were mild, only requiring symptomatic treatment in a few cases (with complete recovery). An important point was the absence of life-threatening systemic reactions and fatal events. Some caution is required in the interpretation of safety data due to a significant heterogeneity between studies mainly related to differences in subjects disease and co-morbidities and in measurement tools and units (mainly regarding local reactions). Moreover, premedication was not used in all clinical trials which may affect the comparative analysis of adverse reactions between studies.
Further studies should concentrate on the following points:(i) it is necessary to standardize accelerated build-up protocols, mainly cluster schedules, regarding type of vaccines, dosage and pharmacologic units, duration, number of injections administered and gaps between increasing doses; (ii) it is also important to establish more appropriate time-points to measure and analyze the outcomes to assess the early effects of the accelerated schedules, including at least one measurement at the end of the build-up phase; (iii) standardization of scoring systems is critical to evaluate the efficacy and safety of SCIT; (iv) finally, further RCTs exclusively enrolling pediatric patients are required to provide a better analysis of efficacy and safety of accelerated immunotherapy schedules in this population.
In conclusion, the current evidence provides support for the efficacy and safety of accelerated SCIT build-up schedules in the treatment of respiratory allergy in pediatric patients.
Conflicts of interestNone declared.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.