Bronchiolitis is a heterogeneous group of diseases of an inflammatory nature, centered on small conducting airways and often associated with other pulmonary disorders. No single classification scheme for bronchiolar diseases has been widely accepted. In this retrospective study, it was decided to apply a new clinical and pathological interpretative classification.
ObjectivesTo propose a new clinical and pathological interpretative classification for adult bronchiolitis, based on statistical analysis of a population of 193 patients with histopathological diagnosis of bronchiolitis.
Materials and methodsA retrospective study analyzed the epidemiological characteristics, co-morbidities and radiological findings present in a group of patients with histopathological diagnosis of bronchiolitis.
ResultsThis trial involved 193 cases collected over a period of eleven years; 48 (24.9%) patients had simultaneous pulmonary disease; non-pulmonary diseases, such as cardiovascular diseases, type II Diabetes mellitus and dyslipidemia were present in 57 cases. The image study was extremely important in order to integrate clinical and pathological aspects.
In this study respiratory bronchiolitis related to smoking dominated. The radiological findings confirmed the secondary nature of the histopathological features, with prevalence of ground-glass patterns, pneumothorax and patterns of interstitial involvement, as described in the literature. It was also verified that clinical behavior of different forms of bronchiolitis was important to distinguish the various types, since they could progress without typical anatomopathological aspects.
ConclusionThis trial showed that the vast majority of diagnosis obtained corresponded to bronchiolitis as secondary to pulmonary pathology. In most cases, morphological findings had to be complemented with clinical and radiological characteristics, in order to obtain the final diagnosis.
Bronchiolitis is a heterogeneous group of inflammatory diseases centered on small conducting airways and often associated with other pulmonary disorders. The etiology is diverse and the different causes are often reflected in particular anatomopathological changes. As a consequence, it is important to evaluate the clinical, radiological, epidemiological and socio-cultural aspects of the patient, to obtain the final diagnosis.1 Pulmonary function tests and plain chest radiography may demonstrate abnormalities. High-resolution CT (HRCT) scanning of the chest is the most important diagnostic tool.2 However, they rarely are sufficient to avoid bronchoscopic, transthoracic or surgical biopsy.
No single classification scheme for bronchiolar diseases has been widely accepted.3,4 Most commonly, classification is performed according to histopathologic patterns or etiology. In this retrospective study, it was decided to apply a clinical and pathological interpretative classification, in order to emphasize the morphology as the basis of clinical and pathophysiological understanding, guiding therapy and prognosis.5
ObjectivesThe epidemiological characteristics, co-morbidities and radiological findings present in a group of patients with histological diagnosis of bronchiolitis were analyzed to validate a clinical and pathological interpretative classification.
Materials and methodsIn order to draw up this study, the individual hospital information of patients with a histopathological diagnosis of bronchiolitis obtained between 2000 and 2011 was consulted. The epidemiological characteristics of the study population were recorded, including age, gender, occupational and inhalation risk, symptoms, place of residence, as well as analysis of the radiological findings, co-morbidities, pulmonary function tests and type of histological diagnosis obtained, according with the clinical and pathological classification reported in Table 1. The fragments for anatomopathological study were obtained by surgical biopsy, trans-thoracic CT guided biopsy or trans-bronchial biopsy. The statistical analysis was conducted using Microsoft Office Excel 2007®.
Bronchiolitis – clinical and pathological interpretative classification proposed by the authors of this original article, based on the results of the retrospective analysis, as well as on a review of the current literature.
| Primary bronchiolar diseases with specific histopathological marker |
|---|
| Acute bronchiolitis |
| ✓ Infections (RSV, Adenovirus, Parainfluenza virus, Mycoplasma, Chlamydia) |
| ✓ Toxic inhalation |
| ✓ Connective tissue disorders |
| ✓ Organ transplantation |
| Constrictive bronchiolitis |
| ✓ Connective tissue disorders |
| ✓ Inflammatory intestinal diseases |
| ✓ Primary biliary cirrhosis (PBC) |
| ✓ Organ transplantation |
| ✓ Drugs (Penicillamine, gold, chemotherapy) |
| ✓ Sarcoidosis |
| ✓ Infections (virus) |
| ✓ Chronic hypersensitivity bronchiolitis |
| ✓ Idiopathic |
| Obliterans/obliterative bronchiolitis |
| ✓ Inhalation acute injury |
| ✓ Hypersensitivity bronchiolitis |
| ✓ Organ transplantation |
| ✓ Idiophatic |
| Diffuse panbronchiolitis |
| ✓ Asiatic type |
| ✓ Systemic eritematous lupus (SEL) |
| Respiratory bronchiolitis |
| ✓ Tobacco |
| ✓ Idiophatic |
| Eosinophilic bronchiolitis |
| ✓ Infectious |
| ✓ Hypersensitivity |
| Granulomatous bronchiolitis |
| ✓ Secondary to systemic chemotherapy |
| ✓ Crohn's disease |
| ✓ Idiophatic |
| Dust airway disease (mineral/non-mineral) |
| Diffuse micro-aspiration bronchiolitis |
| Follicular bronchiolitis |
| ✓ Connective tissue disorders |
| ✓ Infections (AIDS) |
| ✓ Hypersensitivity reactions |
| ✓ Idiopathic |
| Non-specific/chronic bronchiolar diseases |
| Secondary bronchiolar diseases with pulmonary/systemic involvement |
|---|
| Acute bronchiolitis |
| ✓ Infections |
| ✓ Chronic aspiration |
| ✓ Toxic inhalation |
| ✓ Diffuse alveolar damage (DAD) |
| ✓ Connective tissue disorders |
| ✓ Cystic fibrosis infections |
| ✓ Wegener's granulomatosis |
| ✓ Diabetes mellitus |
| ✓ Dyslipidemia |
| Constrictive bronchiolitis |
| ✓ Tobacco |
| ✓ Organ transplantation |
| ✓ Acute bronchiolitis and organizing pneumonia |
| ✓ Pulmonary sarcoidosis |
| ✓ Airway-centered interstitial fibrosis (ACIF) |
| ✓ Carcinoid tumor |
| ✓ Neuroendocrine cell hyperplasia |
| Bronchiolitis obliterans/obliterative |
| ✓ Bronchiolitis obliterans (BO) with organizing pneumonia (OP) |
| ✓ Acute fibrinous organizing pneumonia (AFOP) |
| ✓ Diffuse alveolar damage (DAD) |
| Diffuse panbronchiolitis (SEL) |
| Respiratory bronchiolitis |
| ✓ Respiratory bronchiolitis-associated with interstitial lung disease (RB-ILD) |
| ✓ Desquamative interstitial pneumonia (DIP) |
| ✓ Pulmonary Langerhans’ histiocytosis |
| Eosinophilic bronchiolitis |
| ✓ Acute and chronic eosinophilic pneumonia |
| ✓ OP/COP |
| ✓ COPD |
| Granulomatous bronchiolitis |
| ✓ Tuberculosis and Mycobacterium avium complex infection |
| ✓ Aspergillosis |
| ✓ Primary granulomatous diseases |
| ✓ Pneumoconiosis |
| Follicular bronchiolitis |
| ✓ Connective tissue disorders |
| ✓ Infections (AIDS) |
| ✓ Hypersensitivity reactions |
| ✓ Tuberculosis |
| ✓ Bronchiectasis |
| Chronic dust airway disease (mineral/non-mineral) |
| Diffuse aspiration bronchiolitis |
| ✓ Idiopathic pulmonary fibrosis (IPF) |
| Non-specific/chronic bronchiolar lesions in pulmonary diseases |
| Chronic/lymphocytic bronchiolitis |
Between 2000 and 2011, 193 histological diagnoses of bronchiolitis were confirmed in our hospital. The material for pathologic study was obtained in most cases by surgical biopsy (n=150; 77.7%), as well as by transthoracic CT guided biopsy (n=34; 17.6%). In 9 patients (4.7%) it was obtained by bronchoscopy with transbronchial biopsies. The enrolled population had a mean age of 48.72 (±17.16) years with predominance of males (69.4%). Most of the patients had several co-morbidities, prevailing lung pathology in 48 (28%) of patients. 33 patients had a history of previous spontaneous pneumothorax, 5 had history of COPD and 5 of Asthma. Cardiovascular pathology was also present in 31 (18%) of patients, predominantly arterial hypertension in 28 patients. Endocrine and metabolic disorders were present in some patients, such as type 2 diabetes mellitus in 14 and dyslipidemia in 12 patients.
The histological results obtained were extremely varied, with a predominance of respiratory bronchiolitis related to smoking in 104 patients (53.9%). Distinct forms of bronchiolitis were also observed, such as obliterative bronchiolitis in 31 patients (16.1%), constrictive bronchiolitis in 22 cases (11.4%) and chronic bronchiolitis in 13 (6.7%). Less prevalent histological types such as follicular bronchiolitis (n=9; 4.7%), granulomatous, acute and eosinophilic bronchiolitis were also found. The major epidemiological features of these histological subtypes are listed in Table 2.
Epidemiological features of the most prevalent histopathological types of bronchiolitis.
| Histopathological subtype | Radiological findings | Occupation inhalation risk (n) | Place of residence | Duration of symptoms | Smoking habits |
|---|---|---|---|---|---|
| Respiratory bronchiolitis 53.9% | Pneumothorax – 38.5% Pulmonary nodules and lung masses – 19.2% | 14.3% Locksmith (5) Car mechanic (4) Bricklayer (3) Handicraftsman (1) Painter (1) Factory worker (1) | Rural setting – 47% City setting – 53% Residence nearby industries – 9.6% | <1month – 38.8% >1month – 43.9% No symptoms – 17.3% | Smokers – 80.7% PYa – 14.06±20.16 Ex-smokers – 3% Non smokers – 16.3% |
| Obliterative bronchiolitis 16.1% | Ground glass opacities – 32.1% Densification areas – 17.8% Pulmonary nodules and lung masses – 17.8% Interstitial reticulation – 10.7% | 16% Bricklayer (2) Handicraftsman (1) Locksmith (1) Blower (1) | Rural setting – 68% City setting – 32% Residence nearby industries – 12.9% | <1month – 8% >1month – 88% No symptoms – 4% | Smokers – 20% Ex-smokers – 24% Non smokers – 56% |
| Constrictive bronchiolitis 11.4% | Ground glass opacities – 36.6% Densification areas – 13.6% Interstitial reticulation – 13.3% | 9.5% Bricklayer (1) Carpenter (1) | Rural setting – 52.4% City setting – 47.6% Residence nearby industries – 13.6% | <1month – 14.3% >1month – 81% No symptoms – 4.7% | Smokers – 23.8% Ex-smokers – 9.5% Non smokers – 66.7% |
| Chronic bronchiolitis 6.7% | Ground glass opacities – 36.4% Densification areas – 27.6% | 36.5% Bricklayer (2) Baker (1) Carder (1) Farmer (1) | Rural setting – 72.4% City setting – 27.6% Residence nearby industries – 25% | >1month – 90.9% No symptoms – 9.1% | Smokers – 36.4% Non smokers – 63.6% |
| Follicular bronchiolitis 4.7% | Pulmonary nodules – 50% Ground glass opacities – 37.5% | 14.3% Factory worker (1) | Rural setting – 51.2% City setting – 48.8% Residence nearby industries – 11.1% | >1month – 85.7% No symptoms – 14.3% | Smokers – 14.3% Non smokers – 85.7% |
The group of patients with respiratory bronchiolitis showed a predominance of pulmonary nodules and lung masses (n=20; 19.2%). In 31 (29.8%) patients, diffuse parenchymal lung infiltrates and ground glass centrilobular nodules were also found. This syndrome is referred to as RB-ILD. It is also important to mention that in 40 (38.5%) patients, the radiological study revealed the presence of pneumothorax. Only 11 (10.6%) were women and the mean age was of 36.2 years. Some of the patients had already got a history of previous episodes of pneumothorax and 84 (80.7%) were smokers.
Ground glass opacities (n=10; 32.1%), densification areas (n=6; 17.8%), pulmonary nodules and lung masses (n=6; 17.8%) were some of the patterns observed in the group of patients with obliterative bronchiolitis. There was a predominance of men (23; 74%), mean age of 64.5 years, 17 (56%) were non-smokers and there was no significant history of occupational inhalation exposure.
Patients with constrictive bronchiolitis presented ground glass opacities (n=8; 36.6%), densification areas (n=3; 13.6%) and interstitial reticulation (n=3; 13.6%), results that are similar to the group of patients with chronic bronchiolitis (n=5; 36.4% and n=4; 27.6% respectively). In both groups most of the patients were non-smokers, and there was reference to rural housing. In the first group 11 (50.5%) were male with mean age of 57.1 years, and in the second group there was prevalence of female patients (n=9; 69%) with mean age of 51.1 years.
In the group of patients with follicular bronchiolitis, pulmonary nodules (n=5; 50%) and ground glass opacities (n=4; 37.5%) were prevalent. All patients were women, the mean age was 57.2 years and most of them were non-smokers (n=7; 85.7%).
Unfortunately, it was only possible to obtain the pulmonary function tests of 80 patients (41.4%). This group showed a predominance of males (60%) and had an average age of 51.5 years (±15.9). 33 were non-smokers (41.2%), 40 were active smokers (50%) with a mean smoking burden of 30 PY (±20.2) and 7 were ex-smokers (8.8%).
Spirometry revealed values within the normal range in 55 (69%) patients. In this group, 22 (40%) patients were diagnosed with respiratory bronchiolitis, 10 (18.2%) constrictive bronchiolitis and 8 (14.5%) obliterative bronchiolitis. An obstructive pattern was present in 19 (23.5%), 6 (31.6%) of these patients were diagnosed a respiratory bronchiolitis, also 6 (31.6%) with obliterative bronchiolitis, 4 (21%) with constrictive and 3 (15.8%) with chronic bronchiolitis. A restrictive pattern was present in only 4 (5%). Three were diagnosed with respiratory bronchiolitis and one constrictive bronchiolitis. A mixed defect was detected in 2 (2.5%). Comparing the pulmonary function tests between the smokers and non-smokers, it was found that 12.1% of the non-smokers presented an obstructive pattern in the pulmonary function tests, and in the group of smokers the predominance of this pattern was higher (32.6%).
As spirometry showed mostly values within the normal range (69%), it did not contribute to the diagnosis by itself. Moreover, in this retrospective analysis, only 80 (41.4%) patients performed the pulmonary function tests, which may be a bias in the study.
DiscussionBronchiolitis, a generic term applied to various inflammatory diseases affecting the bronchioles, is the most common form of disease affecting small airways.4 It refers to a large number of clinical entities, with the presence of inflammation and fibrosis which occur predominantly at the membranous segments.7 It constitutes a heterogeneous group of etiologically, clinically, and pathologically disparate lesions.1
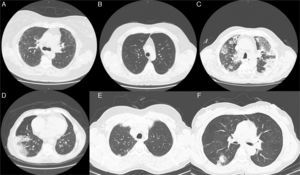
Radiological imaging of the chest, especially HRCT, was demonstrated to be the best tool in the diagnostic evaluation of patients with suspected small airways disease.8,9 Chest radiography demonstrates normal findings or mainly hyperinflation in purely obstructive bronchiolar lesions such as constrictive/chronic bronchiolitis. In other primary bronchiolar disorders, small nodules or reticulonodular infiltrates (Fig. 1A) may be observed.10 Features of bronchiolar disease on HRCT can be broadly categorized into direct and indirect signs.8,9 Direct CT findings of bronchiolar disease include bronchiolar wall thickening, bronchiolar dilatation and luminal impaction that affect airways directly visible in the lung periphery.11 Bronchiolar wall thickening may occur due to inflammation or fibrosis. Bronchiolar luminal impaction with secretions or fibrotic material manifests as nodular and linear branching centrilobular opacities on CT. The “tree-in-bud” pattern represents a form of bronchiolar impaction in which branching linear structures have more than one contiguous branching site.9 Indirect signs of bronchiolar disease on CT include subsegmental atelectasis and air trapping.9,10 In bronchiolar diseases, the mosaic pattern is caused by hypoventilation of alveoli distal to bronchiolar obstruction, which leads to secondary vasoconstriction and is seen on CT scans as areas of decreased attenuation.12,13
Radiological features (Chest CT images) observed in some of the enrolled clinical cases. (A) Image revealing reticularnodular infiltrates, associated with areas of ground glass opacities. These features are consistent with UIP pattern and can be observed in various types of bronchiolitis, such as obliterative, constrictive, chronic and follicular bronchiolitis. (B) Diffuse micronodular pattern usually present in conditions related to tobacco, particularly in cases of respiratory bronchiolitis. A centrilobular emphysema in the upper lobes and thickened bronchial walls are also noted. (C) Extensive parenchymal changes, densification with areas of ground-glass bilaterally and (D) areas of consolidation associated with air bronchogram. These patterns can be found in various types of bronchiolitis, such as obliterative and constrictive bronchiolitis. (E) Subpleural nodule in the left upper lobe which may be associated with respiratory or obliterative bronchiolitis. (F) In the upper segment of the right lower lobe, a grossly nodular densification is observed, which may be present in almost all the histopathological types of Bronchiolitis.
In our sample, radiological findings were varied, and as discussed above, the most common patterns were pulmonary nodules and lung masses in 22.4% of patients (Fig. 1E), ground glass opacities in 18.9% (Fig. 1A, C and F), micronodular pattern or presence of interstitial reticulation (Fig. 1B) in 10.6%, and pneumothorax was present in 23%. As mentioned, a small amount of patients had normal radiological study (2.8%).
No single classification scheme for bronchiolar diseases has been widely accepted. Attempts have been made to classify bronchiolar disorders from different points of view including those of the clinician, pathologist, and radiologist.3,4 Most commonly, classifications are performed according to histopathologic patterns or etiology.14 Early descriptions and clinical distinctions of the bronchiolar diseases appeared around the 70s, with William Thurlbeck and Averill Liebow. With the need to obtain lung tissue to guide the diagnosis and therapy, new studies of bronchial pulmonary diseases emerged. Awareness of the great morphological variability of bronchial diseases, led to several attempts at classification. Nowadays, it is understood that bronchiolar diseases can be primary, often idiopathic, and secondary, associated with other lung diseases. In Table 1, it is described a clinical and pathological interpretative classification of bronchiolitis.
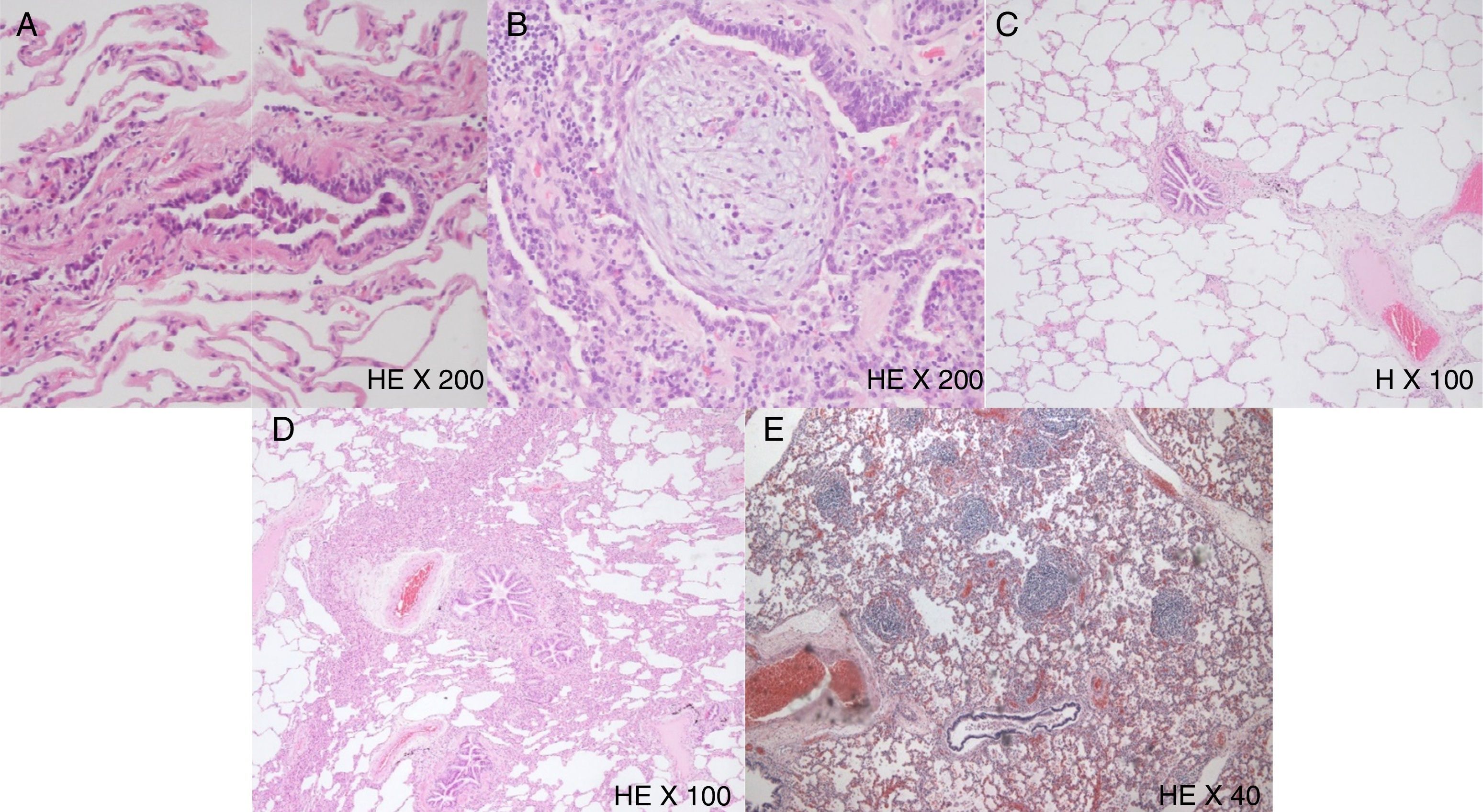
Respiratory bronchiolitis is a separate entity from the other forms of bronchiolitis. It is a clinicopathologic entity seen almost exclusively in current or former cigarette smokers, with various alterations of bronchioles ranging from potentially reversible inflammatory reactions to fixed scarring. It was first described by Niewoehner and colleagues15 as an incidental finding at autopsy of young cigarette smokers. Since this initial report, respiratory bronchiolitis has been recognized as an extremely common histological lesion thought to occur in virtually all cigarette smokers, and the term refers to the universal inflammatory reaction in respiratory bronchioles, known as a smoker's bronchiolitis. It also has been rarely related with other inhalational exposures. The most distinctive feature of respiratory bronchiolitis is the prominent accumulation of pigmented macrophages in the lumen of respiratory bronchioles and adjacent alveoli (Fig. 2A).3 It usually occurs without symptoms or physiologic evidence of lung disease. In a small portion of patients, however, it can cause symptomatic diffuse parenchymal lung infiltrates as well as ground glass centrilobular nodules, especially in upper lobes. This syndrome is referred to as RB-ILD.5 In our sample 31 (29.8%) patients with respiratory bronchiolitis had RB-ILD. This is a clinicopathologic entity seen almost exclusively in current or former cigarette smokers and may be confused with other interstitial lung diseases, such as hypersensitivity pneumonitis and diffuse alveolar hemorrhage. Histologically, RB-ILD is characterized by the presence of pigmented macrophages and mild interstitial inflammatory changes centering on respiratory bronchioles and neighboring alveoli (peribronchiolar air spaces) with sparing of more distal air spaces. The changes are patchy and have a bronchiolocentric distribution.5
Histological features observed in some of the selected patients. (A) Photomicrograph of a respiratory bronchiolitis, with intraluminal pigmented macrophages and parietal constriction with fusiform cells hyperplasia. H&E stain, 200× original magnification. (B) Bronchiolitis obliterans showing fibrinous luminal exudate, accompanied by some neutrophils and progressive fibroblasts and mesenchymal cells proliferation, as well as the typical Masson Body or bronchiolar luminal myofibroblastic inflammatory polyp. H&E stain, 200× original magnification. (C) Photomicrograph illustrating the presence of fibroblast proliferation associated with collagen deposition, as well as alveolar and septal rupture and centrilobular emphysema, resulting in constriction of the airway lumen, which is compatible with constrictive bronchiolitis. H stain, 100× original magnification. (D) Chronic bronchiolitis presenting with characteristic parietal fusiform cells hyperplasia and lymphoplasmacytic infiltration. H&E stain, 100× original magnification. (E) Follicular bronchiolitis represented by lymphocytic germinal centers accompanying small airways. H&E stain, 40× original magnification.
In patients with respiratory bronchiolitis, chest radiographs are usually normal, but in symptomatic patients, treatment with systemic corticosteroids reduces inflammation and improves functional recovery.2 In our sample, there was a predominance of pulmonary nodules and lung masses (19.2%), which is not a regular feature of this entity and does not fit in with the known literature. No explanation was found for this particular result.
Our results demonstrated a strong connection to the presence of pneumothorax in this group of patients. Forty-one were submitted to surgical treatment and the analysis of the surgical biopsy confirmed the diagnosis of respiratory bronchiolitis. 80.7% of the patients were smokers with a mean smoking burden of 14 PY (±20.16). These facts are consistent with the known literature that it is associated with tobacco and they enhance the fact that this type of bronchiolitis should be seen as a separate entity.
Obliterative bronchiolitis runs with bronchiolar changes located to the respiratory bronchioles and alveolar sac, characterized by fibrinous luminal exudate, accompanied by some neutrophils and progressive fibroblasts and mesenchymal cells proliferation (Fig. 2B). These changes can progress to the membranous and muscle bronchioles, settling BOOP/COP progressively. The distribution of lesions is usually focal and heterogeneous, making the diagnosis difficult. Chest radiographs can be normal or a mosaic pattern in the CT scan can be detected.5,16,17
The presented results were consistent with the literature, the ground glass pattern being the most prevalent (32.1%) radiological finding. No relation was found to tobacco smoke exposure, and 88% of the patients had sustained symptoms.
Constrictive bronchiolitis is characterized by a distinctive pattern of peribronchiolar fibrosis ultimately resulting in complete absence of the bronchiolar lumen.3,4,17 The fibrosing inflammatory process surrounds, rather than fills the lumen, resulting in extrinsic compression and obliteration of the airway with apparent epithelial hyperplasia (Fig. 2C). Progressive airway obstruction can be detected by pulmonary function testing.18 Chest radiography can demonstrate normal findings and HRCT demonstrates mosaic areas of decreased attenuation and vascularity, evidence of air-trapping, and peripheral cylindric bronchiectasis.10,11 Constrictive bronchiolitis tends to be progressive and is poorly responsive to corticosteroid therapy.5
The results are similar to those of the obliterative bronchiolitis, the ground glass pattern being the most prevalent (36.6%) radiological finding. No relation was found to tobacco smoke exposure, and 81% of the patients had sustained symptoms. The typical cases obtained in lung transplanted patients were not considered in this study.
Chronic/lymphocytic bronchiolitis is a nonspecific type of airway disease, characterized by infiltration of the large and small airways by lymphocytes, not yet organized into germinal centers (Fig. 2D). This type of airway inflammation may occur in various conditions and has been well described in lung transplant patients, where it might represent an alloreactive injury consequent to allograft rejection.19 Lymphocytic bronchiolitis has also been reported in patients with rheumatoid arthritis, Sjögren's syndrome, and other connective tissue diseases. The outcome of lymphocytic bronchiolitis is not precisely known, with some patients improving on corticosteroids whilst others continue to deteriorate.20 The prognosis of the airway disorder probably depends largely upon its cause and the clinical context.
In the results described, there was prevalence of ground glass opacities as well as reference to occupations with inhaled risk in 36.5% patients, and rural housing in 72.4%. There was no association with transplanted patients or known connective tissue diseases.
Follicular bronchiolitis is defined by the presence of peri-bronchiolar hyperplastic lymphoid follicles with reactive germinal centers distributed along bronchovascular bundles (Fig. 2E). This form of pulmonary lymphoid hyperplasia is a common secondary finding in patients with bronchiectasis, affecting proximal large airways.21,22 In other patients, however, follicular bronchiolitis is a manifestation of primary and idiopathic pulmonary lymphoid hyperplasia. These patients usually present with progressive dyspnea and chest radiographs may sometimes look normal. However, the predominant findings on chest radiography are bilateral, small, nodular or reticulonodular infiltrates, with intrathoracic adenopathies. The cardinal features of follicular bronchiolitis on HRCT consist of centrilobular and peribronchial nodules measuring 1–12mm in diameter, variably associated with areas of ground-glass opacity. Mild bronchial dilatation with wall thickening has been reported in some patients.21 Prognostic implication of follicular bronchiolitis is unclear and the treatment is generally directed to the underlying disease, when identified.
Our results showed that the main radiological finding was pulmonary nodules in 5% of the cases, as well as ground glass opacities in 37.5%. Most of the patients had prolonged symptoms. However, no relation to connective tissue diseases was found, as described in the literature.
ConclusionThe vast majority of the diagnosis obtained corresponded to bronchiolitis secondary to pulmonary pathology, with emphasis on the respiratory bronchiolitis, associated with tobacco. Nevertheless this entity should be evaluated separately from the other histological types, due to its distinct features.
In order to correctly classify the type of bronchiolitis, and make an accurate differential diagnosis with other pathologies, it is essential to integrate all the information obtained from the histopathological analysis, as well as with clinical, epidemiological and radiological characteristics of the patient.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.