Positive results from recent clinical trials led to the approval of immunotherapy based on checkpoint inhibitors (ICI) for a variety of cancers.1 The ICI-class drugs include anti-PD-1 (nivolumab, pembrolizumab), anti-PD-L1 (atezolizumab, durvalumab, avelumab), and anti-cytotoxic T lymphocyte-associated antigen (CTLA-4) agents (ipilimumab, tremelimumab).2 It is important to mention that all trials leading to the approval of CTLA-4 or PD-1/PD-L1 blocking agents excluded patients with pre-existing active autoimmune disorders (AD) from enrolment,1 because of the potential for increased severe toxicity, such as fulminant myocarditis. Having an overactive immune system is the main reason why many patients with cancer and AD have not been included in hundreds of clinical trials of immunotherapy drugs.
Up to 80% of patients under ICI experience inflammatory and immunorelated adverse events (irAEs) in various organs that can be detrimental to patient outcomes.3 The actual mechanism of ICI-induced toxicities remains poorly defined, as does the exact mechanism of several AD.
AD represent a spectrum of conditions with a common denominator: the (auto) immune attack and damage of normal tissues, either localized in individual organs, systems, or widespread (systemic). More than 80 distinct AD have been identified, including some diseases with moderately high prevalence such as rheumatoid arthritis, and inflammatory bowel disease.1 As the use of ICI expands to more types of cancer, the need to determine the risk-benefit ratio in patients with cancer and pre-existing AD is increasing. The number of studies and clinical cases reported is growing. Khan et al. conducted a study in the University of Texas in order to calculate the incidence of AD in the population of lung cancer patients older than 65. Approximately 14% to 25% of the enrolled patients had an AD. The most common AD diseases in the patients studied were rheumatoid arthritis (5.9%), psoriasis (2.8%), and polymyalgia rheumatic (1.8%).4 A more recent paper by Khozin et al.,5 also presents a high value of prevalence of AD in lung cancer patients (22%). Potential explanations included in these articles, for the relatively high rate of AD among patients with lung cancer include advanced age at diagnosis and smoking history, which has been linked to higher risk of certain AD.4
In this context, the authors describe two clinical cases of patients with AD, who were treated with immunotherapy, without adverse effects.
Case 1The authors present the case of a 68-year-old male with a diagnosis of squamous cell carcinoma, initially in stage IIIC (T4N3M0). He had a history of asymptomatic systemic lupus erythematosus, diagnosed at the age of 48. At the time of the lung cancer diagnosis, he was being treated with hydroxychloroquine and corticosteroids for dyslipidaemia, COPD and high blood pressure. The patient was treated with first-line platinum and gemcitabine doublet, after decision in a multidisciplinary meeting, because of the large size of the tumour. Docetaxel was used as second line therapy, after evidence of imagiological progression, and because of the PD-L1 negative expression on tumour cells, Nivolumab was started after disease progression. After four months of immunotherapy, without adverse effects or flare of lupus, imagological confirmation of the progression led to the suspension of therapy.
Case 2A 63-year-old male with a diagnosis of squamous cell carcinoma, initially tumour stage IVA (T2bN3M1a), due to pleural, pulmonary and ganglionic contralateral mediastinal metastasis is described. The patient had history of psoriasis since he was 50 years old, and smoking habits. At the time of the lung cancer diagnosis, his AD was stable only on topical corticotherapy. The patient was treated with first-line platinum and gemcitabine doublet and, after disease progression, because of the PD-L1 negative expression on tumour cells, Nivolumab was started as second line therapy. The patient is currently in the 6th month of immunotherapy, with stability of the imagological findings. During the entire course of treatment with nivolumab, his psoriasis remained stable without the need for systemic immunosuppression.
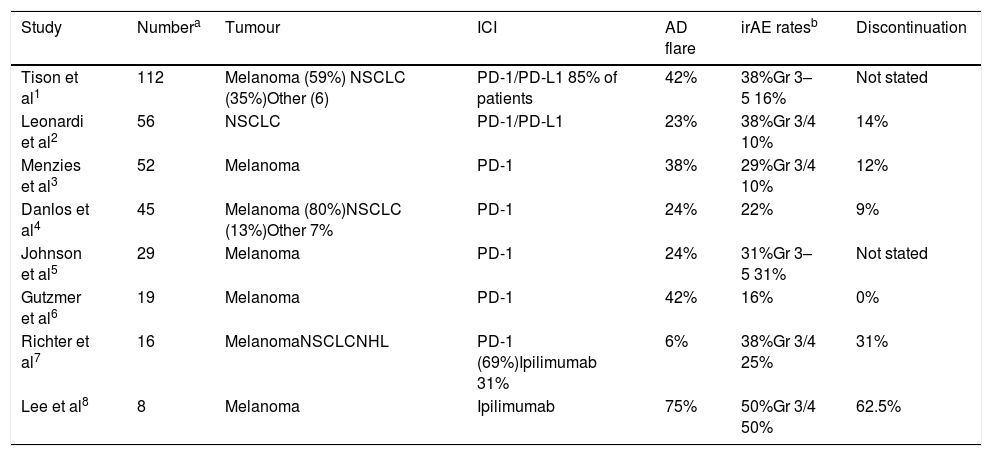
Several published articles have described the use of ICI in patients with AD, similar to the cases reported in this article. (Table 1) In a group of 52 patients with pre-existing AD and advanced melanoma, treated with PD-1 antibodies (either pembrolizumab or nivolumab), twenty (38%) patients flared after a median of 1.3 months after the initiation of PD-1 antibody treatment, including 7/13 with rheumatoid arthritis, 3/3 with polymyalgia rheumatica, 2/2 with Sjogren's syndrome, 1/2 with scleroderma, 2/2 with immune thrombocytopenic purpura, 3/8 with psoriasis, and 1/4 with Graves’ disease.1 Khunger et al., reported two cases of patients with Psoriasis, who were treated with Nivolumab for up to 6 months, without worsening of the underlying condition.
Some retrospective case series evaluating patients with AD and ICI use. Adapted from Laura et al.
| Study | Numbera | Tumour | ICI | AD flare | irAE ratesb | Discontinuation |
|---|---|---|---|---|---|---|
| Tison et al1 | 112 | Melanoma (59%) NSCLC (35%)Other (6) | PD-1/PD-L1 85% of patients | 42% | 38%Gr 3–5 16% | Not stated |
| Leonardi et al2 | 56 | NSCLC | PD-1/PD-L1 | 23% | 38%Gr 3/4 10% | 14% |
| Menzies et al3 | 52 | Melanoma | PD-1 | 38% | 29%Gr 3/4 10% | 12% |
| Danlos et al4 | 45 | Melanoma (80%)NSCLC (13%)Other 7% | PD-1 | 24% | 22% | 9% |
| Johnson et al5 | 29 | Melanoma | PD-1 | 24% | 31%Gr 3–5 31% | Not stated |
| Gutzmer et al6 | 19 | Melanoma | PD-1 | 42% | 16% | 0% |
| Richter et al7 | 16 | MelanomaNSCLCNHL | PD-1 (69%)Ipilimumab 31% | 6% | 38%Gr 3/4 25% | 31% |
| Lee et al8 | 8 | Melanoma | Ipilimumab | 75% | 50%Gr 3/4 50% | 62.5% |
Excludes immunotherapy-related events that were felt to be an exacerbation of the patient's underlying autoimmune disorder.
1 – Tison A, Quere G, Misery L, et al. OP0196 safety and efficacy of imune checkpoint inhibitors in patients with cancer and preexisting autoimmune diseases: a nationwide multicenter retrospective study [abstract]. Ann Rheum Dis 2018;77:Abstract 147.
2 – Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–1912.
3 – Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–376.
4 – Danlos FX, Voisin AL, Dyevre V, et al. Safety and efficacy of antiprogrammed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018;91:21–29.
5 – Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–240.
6 – Gutzmer R, Koop A, Meier F, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 2017;75:24–32.
7 – Richter MD, Pinkston O, Kottschade LA, et al. Brief report: cancer immunotherapy in patients with preexisting rheumatic disease: the Mayo Clinic experience. Arthritis Rheumatol 2018;70:356–360.
8 – Lee B, Wong A, Kee D, et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann Oncol 2016;27: 1174–1177.
Leonardi et al.6 also conducted a study in 56 patients with NSCLC and AD who received a PD-L1 inhibitor. A total of 55% of patients developed an AD flare and/or an irAE. Exacerbation of the AD occurred in 13 patients. Immune-related adverse events occurred in 21 patients (38%). PD-L1 therapy was permanently discontinued in eight patients because of irAEs.
Kennedy et al. summarized the 8 largest retrospective case series that specifically evaluated patients with pre-existing autoimmunity who underwent treatment with ICIs. The patients in these case series had a wide variety of well-controlled AD, making it difficult to draw conclusions about safety regarding a specific disorder. irAEs and autoimmune exacerbations occurred in a minority of patients.2
Based on the limited safety data from the published case series, recommendations regarding which patients may be appropriate for considering ICI therapy in the setting of pre-existing AD were made.7 Patients with neurologic AD, such as myasthenia gravis, or life-threatening AD should not be considered candidates for ICI therapy. Patients receiving high levels of immunosuppression should also be approached with caution. Patients with pre-existing AD who could be considered for ICI treatment include those with a non-life-threatening AD, who have good control on either no immunosuppression or relatively low levels of immunosuppressive therapy.
It is recommended that a multidisciplinary team should be involved in the decision to initiate ICI therapy, with close monitoring and clear consideration of the severity of the underlying autoimmunity, prognosis of cancer, alternative therapeutic options, and a clear understanding of patients’ preferences with respect to the risks and benefits of the various options. Finally, it is important to continue to expand understanding of the pathogenesis of irAEs and improve the ability to predict and manage irAEs.
Conflicts of interestThe authors have no conflicts of interest to declare.