Arising in China in the winter of 2019, COVID-19 (caused by the SARS-CoV-2 virus) has caused a global pandemic and severely stressed medical systems across the world.
Although knowledge about this novel coronavirus is still emerging, the most common reason for hospitalization of COVID-19 patients is severe respiratory distress.1
COVID-19 has been accurately described as the cause for a proinflammatory and hypercoagulable state with marked elevations seen in Lactate Dehydrogenase, Ferritin, C-reactive protein, D-Dimer, and Interleukin levels.2
The inflammatory response, including production of inflammatory cells and cytokines, induces a procoagulant effect and diffuse endothelial damage that predisposes thrombotic vascular lesions and Disseminated Intravascular Coagulation (DIC).3
D-Dimer is related to the severity of the disease and an increased value is associated with the worst. prognosis. Retrospective studies demonstrated that patients admitted to Intensive Care Unit (ICU) had an elevated D Dimer value and, in this setting, some Authors recommended a therapeutic heparin doses for the patients with higher values.4
A recent ICU observation reported an increased risk of Pulmonary Embolism (PE) in COVID-19 compared to the historical control group even in patients that had undergone the Low Molecular Weight Heparin (LMWH) prophylaxis.5
We evaluated 138 patients with COVID 19 admitted to our Institution between March 2020 and May 2020. All patients were COVID 19 positive according to clinical diagnostic criteria reverse-transcription–polymerase chain-reaction (RT-PCR) and Chest Thoracic tomography. On admission, most of them were haemodynamically stable (78%) and febrile (87%). During hospitalization, some developed progressive respiratory failure and received oxygen supplementation (41%). Four of them were started on Continuous Positive Airways Pressure (CPAP) but two died because of worsening Respiratory Failure.
All patients were treated with hydroxycloroquine (400 mg/day), darunavir/ritonavir (800/100 mg/day) and enoxaparin (4000 UI/day). Some patients (26 pts) received additional therapy with IL-6 and IL-1 antagonist (20%). Every three days after their hospitalization, laboratory exams with inflammatory and coagulation parameters (INR, activated partial thromboplastin time, platelets count, fibrinogen, D-Dimer) were repeated.
In patients with progressive elevation of D-Dimer of over three times the normal value (from 1822 to 5911 μg/mL), we performed a Computed Tomography Pulmonary Angiography (CTPA) and a Doppler Ultrasound (DU) of the lower limbs. The tests were done during the second week of their hospitalization (12,3 ± 3,2 days).
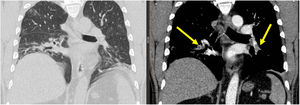
We identified eleven patients with high D-Dimer value, nine of them (6,7%) had signs of Pulmonary Artery Thrombosis (PAT) without Deep Venous Thrombosis (DVT). The lesions were distributed bilaterally at the lower arterial branches (Figure 1). None of the nine patients experienced an objective respiratory worsening and those in oxygen therapy (5 patients) maintained a constant flow. Therapeutic anticoagulation was started with subcutaneous enoxaparin (1 mg/kg) twice daily, followed by warfarin. All patients were discharged home: length of hospital stay (LOS) 21,1 ± 3,7 days.
In Table 1 the laboratory parameters were compared between patients with and without PAT: five days after admission, only D-Dimer value and lymphocyte count were significatively different between the two groups.
Laboratory parameters in patients with Pulmonary Artery Thrombosis (PAT) and the patients without PAT.
| All Pts admission | PAT pts admission | All pts 5 days | PAT pts 5 days | All Pts 10 days | PAT pts 10 days | |
|---|---|---|---|---|---|---|
| C-Reactive Proteine (mg/l) | 54,6 ± 58,1 | 54,6 ± 58,1 | 44,9 ± 65,1 | 54,6 ± 58,1 | 27,0 ± 25,5 | 49,5 ± 27,6 |
| Lymphocite (mmc) | 1294 ± 761,3 | 1260 ± 414,5 | 1465 ± 657,6 | 1075 ± 219,2* | 1762 ± 858,6 | 1290 ± 336,7* |
| Platelets (mmc) | 250,000 ± 84,000 | 263,000 ± 147,000 | 300,000 ± 114,000 | 252,800 ± 52,900 | 352,300 ± 102,900 | 289,000 ± 113,000 |
| Fibrinogen (mg/dl) | 432,3 ± 114,1 | 530 ± 121,6 | 428,5 ± 138,2 | 426,0 ± 14,1 | 379,6 ± 100,4 | 466,1 ± 142,1 |
| LDH (U/l) | 264,4 ± 101,3 | 326,2 ± 121,9 | 295,2 ± 120,6 | 314,5 ± 37,5 | 264,0 ± 73,5 | 309 ± 63,7 |
| INR | 1,2 ± 0,1 | 1,1 ± 0,01 | 1,2 ± 0,15 | 1,05 ± 0,1 | 1,2 ± 0,1 | 1,1 ± 0,1 |
| D-Dimer Ug/l | 465,1 ± 121,7 | 627,4 ± 178,6 | 538,3 ± 102,8 | 2143,6 ± 327,5* | 576,9 ± 169,1 | 1764,9 ± 227,5* |
Parameters are expressed as mean ± SD and statistically evaluated by Student T test. P < 0,05 was considered statistically significant.
Coagulopathic disorders are significantly increased in COVID-19 patients, especially among those with severe disease. Several mechanisms combine systemic inflammation with alterations of coagulation in COVID 19 patients.6
In severe or critically ill patients, the endothelial cells are damaged and release a large amount of inflammatory mediators that may predispose vascular thrombosis. A study performed in ICU setting reported an increased risk of Pulmonary Embolism (PE) in COVID-19 patients treated with Low Molecular Weight Heparin (LMWH) prophylaxis compared to the historical COVID 19 negative control group.7 Another Study reported that the prevalence of Venous Thromboembolism Events (VTE) was higher in ICU compared to general wards patients: 47% vs 3%.8
High blood values of the procoagulant factor levels including fibrinogen and D-dimers have been associated with the worst prognosis and higher mortality.
Kaminetzky and coworkers compared the results of a cohort of 62 patients who underwent CTPA for suspected PE prior to the first case of COVID 19, with 62 patients COVID 19 positive.9 CTPA was positive for PE in 37% of COVID 19 patients (14,5% in pre COVID patients), D-Dimer was associated with a higher prevalence of thromboembolic events and correlated with the degree of PE severity.
In a group of patients admitted to non-ICU wards, DU failed to detect DVT independently of the severity of their condition and length of in-hospital bed rest. The Authors observed that this is apparently in contrast with the relatively frequent reports of PE in hospitalized COVID-19 patients It is possible that local thrombi in the lungs may be the cause of pulmonary arterial manifestations.10, 11
In this paper we reported COVID-19 patients with interstitial pneumonia admitted in our non-ICU Ward.
During the course of hospitalization, in eleven of them, we observed a progressive increase of D-Dimer over three times the normal value, associated with low or normal values of other coagulation or inflammatory blood parameters (CRP, LDH, Ferritin, fibrinogen, INR, aPTT).
Nine CTPA demonstrated a distal thrombosis of the lower pulmonary arterial branches. The mainly basal localization where the pulmonary inflammation is most diffuse and the loss of signs of DVT may suggest a pulmonary thrombosis rather than an embolism.
It is noteworthy that no patients had any signs of respiratory worsening and some of them did not receive oxygen therapy and they were breathing room air.
In conclusion, we described patients with moderate disease who developed a pulmonary vascular injury strictly correlated with an elevation of D-Dimer values. This parameter may help clinicians in identifying COVID-19 stable patients at risk of concurrent pulmonary artery thrombosis.
Further studies will need to better define the meaning of these preliminary observations.
Conflicts of interestThe authors have no conflicts of interest to declare.