Allergic bronchopulmonary aspergillosis (ABPA) is caused by a hypersensitivity response of type 2 T helper (Th2) lymphocytes to antigens, mostly Aspergillus fumigatus (A. fumigatus).1 This abnormal host response to A. fumigatus in a subset of patients with asthma or cystic fibrosis (CF) is likely due to genetic susceptibility that predisposes patients to the risk of developing ABPA. This is characterised by an increased total IgE and Aspergillus-specific IgE, IgG, and IgA in the serum and bronchoalveolar lavage.2 The International Society for Human and Animal Mycology (ISHAM) and the Rosenberg–Patterson criteria are most often used to diagnose ABPA.3,4
Omalizumab is a humanized monoclonal anti-IgE antibody with licensed indication in severe allergic asthma. There are clinical studies that have also shown benefit in the use of omalizumab in ABPA patients without and with cystic fibrosis.5,6 However, the safety and efficacy of omalizumab in ABPA needs further evidence.
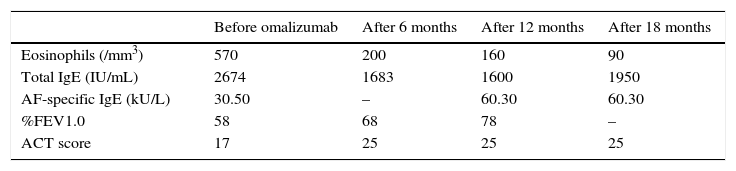
The authors described a 45-year-old caucasian man with long standing atopic asthma with frequent exacerbations, and ABPA diagnosed at the age of 42 years. CF was excluded. At the time of ABPA diagnosis, his predicted forced expiratory volume in 1s (FEV1) was 58% and after bronchodilator was 62%. All Rosenberg–Patterson criteria were met, including central bronchiectasis with pulmonary infiltrates in all lobes evidenced in a thoracic CT scan. Peripheral blood eosinophils were 570/mm3 under systemic steroids, total IgE was 2674IU/mL and AF-specific IgE was 30.50kU/L. No precipitating antibody against AF antigen was found. The patient was treated with prednisolone 40mg/day, inhaled salmeterol/fluticasone propionate combination 50/500mcg twice a day and montelukast 10mg/day. We could not taper the prednisolone dose because of frequent exacerbations.
Oral itraconazole was not administered because the patient was taking atorvastatin (since a previous cerebral stroke), and also because of his history of hepatotoxicity due to azathioprine (which he has been prescribed in the past for his severe dermatitis).
Despite the optimising treatment, frequent exacerbations of his pulmonary disease occurred. So, one year after ABPA diagnosis the patient began treatment with omalizumab 600mg subcutaneously every 2 weeks (the maximum recommended dosage; dose and dose frequency were based on the patient's weight and on pre-treatment serum IgE IU/mL). The authors evaluated the frequency of exacerbations, the Asthma Control Test score (ACT), lung function, peripheral blood eosinophils, total IgE, and blood AF-specific IgE.
Following 6 months of omalizumab, improvements were observed in lung function (FEV1 68%) and the ACT changed from 21 to 25. Peripheral blood eosinophils reduced from 570/mm3 to 200/mm3, total IgE decreased from 2286 to 1683IU/mL. Prednisolone was reduced to 10mg/day.
After 12 months, ACT remained stable, total IgE was 1600IU/mL, peripheral blood eosinophils decreased to 160/mm3, AF-specific IgE raised to 60.30kU/L. Prednisolone was stopped. FEV 1 (%) improved to 78, and a negative bronchodilator response maintained.
At 18 months of treatment, total IgE was 1950U/mL, blood eosinophils decreased to 90/mm3, AF-specific IgE had not changed and Aspergillus precipitins were not detected. (Table 1).
Changes in laboratory and clinical findings after omalizumab treatment.
| Before omalizumab | After 6 months | After 12 months | After 18 months | |
|---|---|---|---|---|
| Eosinophils (/mm3) | 570 | 200 | 160 | 90 |
| Total IgE (IU/mL) | 2674 | 1683 | 1600 | 1950 |
| AF-specific IgE (kU/L) | 30.50 | – | 60.30 | 60.30 |
| %FEV1.0 | 58 | 68 | 78 | – |
| ACT score | 17 | 25 | 25 | 25 |
ACT=Asthma Control Test; AF=Aspergillus fumigatus; FEV1.0=forced expiratory volume in 1 second; Ig=immunoglobulin.
There were no exacerbations or hospitalizations during the whole follow-up period.
There were no adverse events reported during treatment with omalizumab.
The authors present the case of a patient with severe asthma and steroid-dependent ABPA who was successfully weaned from systemic steroid therapy using omalizumab. Corticosteroid-sparing effect of omalizumab is a clinically relevant finding in this case, with a major improvement in asthma symptoms and a reduction in the inflammatory markers: blood eosinophilia and total serum IgE (Table 1). It is not known if the reduction in total serum IgE and eosinophilia is part of the natural course of ABPA or a marker for successful treatment with omalizumab. Lin et al.,7 also suggest that serum IgE in patients treated with omalizumab is still an important marker of disease activity. Mummadj et al.8 showed that in patients with severe asthma treated with omalizumab serum IgE concentration may have clinically significant variability over time, affecting dosing.
The time that omalizumab treatment has to develop a response suggests that IgE antibodies might have an important role in airflow obstruction in ABPA. The serum free IgE are reduced in a dose-dependent manner within two hours of administration of omalizumab.
In our patient a significant FEV1 improvement was achieved, with only a mild bronchial obstruction remaining. Initial studies with omalizumab showed that, although in patients with asthma the exacerbation rate declines during treatment, the effects on lung function remain relatively small.9 More recently, some studies have shown that anti-IgE treatment may prevent or reverse, directly and/or indirectly, airway remodelling changes.10 The effects of omalizumab on preventing the initiation and further propagation of the allergic inflammation cascade may explain why in our patient the improvement in the FEV1 exists, despite the relative stability after 6 months of the total IgE.
In conclusion, omalizumab was effective in reducing exacerbations and the need for systemic corticosteroids as well as in improving asthma symptoms, in a patient with asthma and ABPA who had previously shown an unsatisfactory response to corticosteroids.
Treatment with omalizumab should be considered in asthmatic patients with ABPA who require prolonged use of oral steroids or have frequent relapses of the disease.
Further double-blind, randomised, placebo-controlled studies are necessary to clarify the role of anti-IgE treatment in ABPA.
Conflicts of interestThe authors have no conflicts of interest to declare.