International guidelines define significant bronchodilator response as absolute and percentage change from baseline in forced expiratory volume (FEV1) in the first second and/or forced vital capacity (FVC) ≥12% and 200mL. However, bronchodilator effects on other lung function parameters have also been correlated to some degree of reversible airflow limitation.
ObjectivesTo determine whether changes in other lung function parameters apart from FEV1 and FVC detect functional responses to bronchodilator in asthmatic patients.
Materials and methodsSpirometry and body plethysmography were performed at baseline conditions and after administration of 400μg of salbutamol by metered-dose inhaler through a space chamber device in asthmatic patients. Paired t-tests were used to compare lung function parameters between those with and without criteria for reversibility of airway obstruction according to ATS/ERS criteria. Cut-off values were obtained from the corresponding ROC curves. Measurements evaluated were FEV1, FVC, maximum mid-forced expiratory flow (FEF25–75%), residual volume (RV), inspiratory capacity (IC), airway resistance (Raw) and specific airway conductance (sGaw).
ResultsFrom a total of 100 consecutive asthmatics patients (46% of them men; average age 58.7±14.1 years; 76% with mild to moderate obstruction), 50 patients had a significant bronchodilator response. All of these had noteworthy variations (p<0.004) in PEF, FEF25–75%, RV, Raw and sGaw. The most accurate in predicting a significant bronchodilator response were the absolute and percentage improvements in PEF (≥0.4L/s and 8%), FEF25–75% (≥0.087L/s and 27%) and the percentage of sGaw compared with that at baseline (≥25%). Based on these cut-off values, a sizeable number of the patients defined as non-responders had important changes in airway caliber. 17 patients had significant increments in the percentage of PEF and 10 had changes in absolute volume; 6 patients had increments in percentage and 16 in absolute change of FEF25–75%; 22 patients had increments in the percentage change of sGaw.
ConclusionsChanges of FEV1 and/or FVC may underestimate significant functional response to bronchodilators in asthmatic patients with airway obstruction when considering the change in other lung function parameters.
Airway obstruction reversibility, evaluated by the bronchodilator response, is routinely assessed to assist and support the diagnosis of asthma.1,2 The most recent guidelines published by the American Thoracic Society and the European Respiratory Society (ATS/ERS) in 2005 on reversibility testing define significant bronchodilator response as a 12% per cent increase from baseline and a 200mL absolute increase in forced expiratory volume in 1s (FEV1) and/or forced vital capacity (FVC).3
However, there is considerable variation within the guidelines as to the degree of reversibility considered significant. The British Thoracic Society,4 for example, recommends at least 15% increase of baseline FEV1 while the Global Initiative for Asthma (GINA)2 recommends at least 12% of baseline FEV1 for the bronchodilator response to be considered positive.
Airway obstruction reversibility is still a controversial topic also because there is still a lack of consensus on which variables should be used to express bronchodilator response. In fact, despite the general use of FEV1 or FVC criteria, some studies have concluded that changes in these measurements can frequently underestimate significant responses to bronchodilator in both adults and children.5–12
On the other hand, when reversibility is expressed by the percentage increase in FEV1, it shows bronchodilator responses more frequently in the most severely obstructed patients.13,14
Also, when changes in FEV1 are not meaningful, alternative criteria such as decrease in lung hyperinflation, can establish a substantial response.3,5,7 In the same way, absolute changes in peak expiratory flow (PEF) have proved to be a good substitute to establish airway obstruction reversibility in asthma.15
Difficulties in performing a forced expiratory maneuver may further limit use of FEV1 or FVC, particularly in children and older patients. In these cases, criteria such as airway resistance (Raw) or specific airway conductance (sGaw) may be useful.16,17
Assuming that the significant changes in other ventilatory parameters can explain the bronchodilator improvement of dyspnea even without significant changes in FEV1 and/or FVC, we performed the present study to investigate alternative criteria of positivity in a population of asthmatic patients with documented airway obstruction. Baseline and post-bronchodilator spirometry and body plethysmography were performed on all patients. Unusual pulmonary function variables – PEF, maximum mid-forced expiratory flow (FEF25–75%), total lung capacity (TLC), residual volume (RV), inspiratory capacity (IC), Raw and sGaw – which could possibly assess bronchodilator response were retrospectively collected and analyzed.
MethodsSubjectsOne hundred never-smoker asthmatic patients referred to our Pulmonary Physiology Laboratory by their immunoallergology or pulmonology physician were included in the present study. This population included two matched groups of 50 consecutive patients with and without a significant bronchodilator response defined according to the 2005 ATS/ERS guidelines. Patients under 20 years old, smokers, with severe asthma, recent asthma acute exacerbations or cardiovascular disease, were excluded.
Lung function measurementsSpirometric measurements were made using a pneumotachograph (MasterScreen PTF Jaëger®). Plethysmography measurements were obtained through a body plethysmograph (MasterScreen Body Jaëger®). All spirometric and plethysmographic tests were performed according to accepted standards as recommended by the ATS.18,19
Reversibility testingAll medication likely to interfere with bronchomotricity was previously suspended.18 Short- and long-acting β-agonists bronchodilators were suspended 4h and 12h prior to the test, respectively, while oral theophylines were stopped 12–24h before. Patients were instructed not to smoke and avoid food containing caffeine (coffee, tea, cola) or theobromine (chocolate) for at least 1h before the test.
All patients underwent spirometric and lung volumes evaluation at baseline conditions. An obstructive ventilatory defect was defined by a FEV1/FVC ratio less than 0.70. Only those with verified airflow obstruction and three satisfactory records of FEV1, FVC and PEF were submitted to further challenge with bronchodilator. Airway-obstruction reversibility was tested 10min after administration of four equal and separate doses of 100μg (total dose 400μg) of salbutamol given by a metered-dose inhaler connected to a space chamber device.20,21
Statistical analysisData analysis was performed by IBM SPSS® for Windows version 20.0. All patient demographic and clinical features were reported using frequency and descriptive analyses. Average and standard deviation (SD) scores were calculated for numerical variables, and number and percent for categorical variables. Pearson's chi-square and ordinary t-tests were used for comparison of means and proportions. Two-tailed p values of less than 0.05 were considered to indicate statistical significance. Paired t-tests were used to compare lung function parameters between those with and without criteria for reversibility of airway obstruction according to ATS/ERS criteria. Cut-off values were obtained from the exact value where there was ideal matching of both sensitivity and specificity values on ROC curves. Measurements evaluated were FEV1, FVC, PEF, FEF25–75%, RV, IC, Raw and sGaw.
ResultsDemographicsDemographic data of the studied population are shown in Table 1. Compared to patients with a positive bronchodilator response, patients with no airway obstruction reversibility were older (62.1 versus 56.1 years old) and predominantly female, but these differences did not reach statistical significance. Body mass index was basically the same between both sets of asthmatic patients.
Demographic and lung function characteristics of patients with asthma.
| Reversibility of airway obstructiona | p value | ||
|---|---|---|---|
| No (n=50) | Yes (n=50) | ||
| Gender, n (%) | 0.045 | ||
| Male | 18 (36) | 28 (56.0) | |
| Female | 32 (64.0) | 22 (44.0) | |
| Age, years | 61.2±11.9 | 56.1±15.6 | NS |
| BMI, kg/m2 | 28.4±4.6 | 28.5±5.7 | NS |
| FEV1/FVC | 0.6±0.1 | 0.6±0.1 | 0.029 |
| FVC | |||
| Baseline, L | 2.8±1.0 | 3.1±1.0 | NS |
| Baseline, % of predicted | 99.5±17.9 | 92.2±16.0 | 0.033 |
| Post-bronchodilator, L | 2.8±1.1 | 3.4±1.0 | 0.007 |
| Post-bronchodilator change, L | 0.0±0.3 | 0.3±0.2 | 0.000 |
| Post-bronchodilator change, % | 1.4±3.4 | 10.8±6.2 | 0.000 |
| FEV1 | |||
| Baseline, L | 1.8±0.7 | 1.9±0.7 | NS |
| Baseline, % of predicted | 76.2±16.1 | 67.6±15.9 | 0.008 |
| Post-bronchodilator, L | 1.9±0.7 | 2.1±0.8 | 0.044 |
| Post-bronchodilator change, L | 0.1±0.1 | 0.3±0.2 | 0.000 |
| Post-bronchodilator change, % | 3.9±5.1 | 18.5±11.8 | 0.000 |
| PEF | |||
| Baseline, L/s | 4.7±1.7 | 4.8±1.6 | NS |
| Baseline, % of predicted | 73.1±22.1 | 67.0±16.7 | NS |
| Post-bronchodilator, L/s | 5.0±1.9 | 5.7±1.7 | NS |
| Post-bronchodilator change, L/s | 0.3±0.5 | 0.8±0.5 | 0.000 |
| Post-bronchodilator change, % | 5.6±10.9 | 19.1±12.6 | 0.000 |
| FEF25–75% | |||
| Baseline, L/s | 0.8±0.5 | 0.8±0.5 | NS |
| Baseline, % of predicted | 27.6±15.0 | 23.8±11.9 | NS |
| Post-bronchodilator, L/s | 0.9±0.6 | 1.1±0.7 | NS |
| Post-bronchodilator change, L/s | 0.5±0.2 | 0.3±0.3 | 0.009 |
| Post-bronchodilator change, % | 5.3±23.9 | 39.4±62.6 | 0.001 |
| TLC | |||
| Baseline, L | 5.5±1.3 | 6.0±1.4 | NS |
| Baseline, % of predicted | 108.3±14.3 | 106.9±14.1 | NS |
| Post-bronchodilator, L | 5.5±1.2 | 6.0±1.3 | NS |
| Post-bronchodilator change, L | 0.0±0.2 | 0.0±0.3 | NS |
| Post-bronchodilator change, % | 0.1±4.6 | 1.0±5.4 | NS |
| RV | |||
| Baseline, L | 3.1±4.7 | 2.7±0.8 | NS |
| Baseline, % of predicted | 122.8±31.64 | 132.0±31.70 | NS |
| Post-bronchodilator, L | 2.9±3.7 | 2.4±0.7 | NS |
| Post-bronchodilator change, L | −0.2±1.0 | −0.2±0.3 | NS |
| Post-bronchodilator change, % | −2.7±10.6 | −7.2±11.2 | 0.037 |
| IC | |||
| Baseline, L | 2.5±0.8 | 2.5±0.8 | NS |
| Baseline, % of predicted | 119.6±25.7 | 102.0±24.6 | 0.000 |
| Post-bronchodilator, L | 2.5±0.8 | 3.0±1.8 | NS |
| Post-bronchodilator change, L | 0.1±0.2 | 0.5±1.8 | NS |
| Post-bronchodilator change, % | 4.2±9.2 | 11.2±15.2 | 0.006 |
| Raw | |||
| Baseline, kPas/L | 0.5±0.2 | 0.5±0.2 | NS |
| Baseline, % of predicted | 164.8±78.5 | 170.8±79.9 | NS |
| Post-bronchodilator, kPas/L | 0.4±1.2 | 0.3±0.2 | 0.000 |
| Post-bronchodilator change, kPas/L | −0.1±0.1 | −0.2±0.1 | 0.004 |
| Post-bronchodilator change, % | −15.6±18.5 | −30.3±20.4 | NS |
| sGaw | |||
| Baseline, 1/(kPas) | 0.7±0.3 | 0.7±0.3 | NS |
| Baseline, % of predicted | 77.3±37.8 | 70.9±39.1 | NS |
| Post-bronchodilator, 1/(kPas) | 0.9±0.5 | 1.0±0.5 | NS |
| Post-bronchodilator change, 1/(kPas) | 0.2±0.3 | 0.4±0.3 | 0.002 |
| Post-bronchodilator change, % | 28.7±27.9 | 65.4±54.2 | 0.000 |
Reversibility of airway obstruction defined according to the ATS/ERS criteria of 2005. BMI: body mass index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow; FEF: forced expiratory flow; TLC: total lung capacity; RV: residual volume; IC: inspiratory capacity; Raw: airway resistance; sGaw: specific airway conductance; NS: not significant.
Lung function characteristics between bronchodilator responders and non-responders are compared in Table 1. No meaningful variations could be found neither among any of the spirometry nor plethysmography baseline values or among the majority of the baseline percentage values of predicted between the two groups. There was a substantial difference between responders and non-responders regarding the baseline percentage values of predicted of FEV1, FVC and IC, with bronchodilator responders exhibiting significantly higher values among these particular variables.
After the administration of a short-acting bronchodilator, there were important differences between the absolute change of FVC, FEV1, PEF and IC in the two groups. Also, significant percentage increases were observed in all these variables among the responder group. Absolute and percentage decreases occurred in both RV and Raw that were significantly greater in the responder group, but statistical power was found only for the absolute change of Raw and the percentage change of RV.
Other changes were also important, like the percentage change of FEF25–75% and the absolute change of TLC.
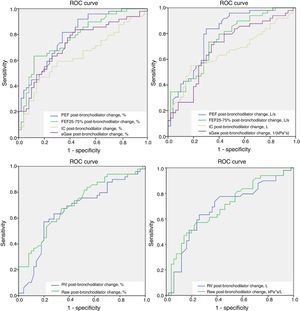
Alternative criteriaThe area under the ROC curve (AUC) was significant for the following criteria: percentage change of PEF, FEF25–75% and sGaw (0.795, 0.779 and 0.731, respectively), and absolute change of PEF and FEF25–75% (0.793 and 0.747, respectively). The AUC for the absolute and percentage changes of both RV and IC, and for the absolute change of sGaw were not significant. Fig. 1 and Table 2 display graphic and numerical representation, respectively, of AUC values for alternative ventilatory criteria.
Receiver operating characteristic (ROC) curves were used to assess alternative pulmonary function tests variables that could define a positive bronchodilator response in a population of asthmatic patients. The area under the ROC curve (AUC) was significant (>0.070) for the following criteria: percentage change of PEF, FEF25–75% and sGaw; and absolute change of PEF and FEF25–75%. Cutt-off values were attained from the exact curve speck were sensitivity and specificity reached the best correlation: absolute PEF change ≥0.4L/s; percentage PEF change ≥8%; absolute FEF25–75% change ≥0.087L/s; percentage FEF25–75% change ≥27%; and percentage sGaw change ≥25%. PEF: peak expiratory flow; FEF25–75%: maximum mid-forced expiratory flow; IC: inspiratory capacity; Raw: airway resistance; sGaw: specific airway conductance.
Area under the ROC curve (AUC) for alternative lung function criteria to define positive bronchodilator responses.
| Variable | AUC |
|---|---|
| PEF, absolute change | 0.793 |
| PEF, % change | 0.795 |
| FEF25–75%, absolute change | 0.747 |
| FEF25–75%, % change | 0.779 |
| IC, absolute change | NS |
| IC, % change | NS |
| Raw, absolute change | NS |
| Raw, % change | NS |
| sGaw, absolute change | NS |
| sGaw, % change | 0.731 |
PEF: peak expiratory flow; FEF: forced expiratory flow; IC: inspiratory capacity; Raw: airway resistance; sGaw: specific airway conductance; NS: not significant.
Since PEF is a parameter that depends on the individual ability to perform a forced expiratory maneuver, a correlation analysis between this variable and sGaw, an independent parameter, was undertaken and it showed a linear relationship between the two variables (R=5.7).
ROC analysis, specifically the best match point between sensitivity and specificity, defined the following cut-off values for the lung function variables with significant AUC: an absolute and percentage change of PEF ≥0.4L/s and ≥8%; an absolute and percentage change of FEF25–75% ≥0.087L/s and 27%; and a percentage change of sGaw ≥25%. The sensitivity and specificity values for each of these new variables were as follows: 83.7% and 70.0% for the absolute PEF change; 81.6% and 66.0% for the percentage PEF change; 74.0% and 68.0% for the absolute FEF25–75% change; 60% and 88.0% for the percentage change of FEF25–75%; and 84.0% and 56.0% for the percentage sGaw change.
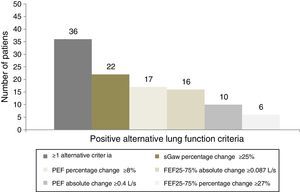
Based on the cut-off values defined by ROC analysis, it was found that a large part of the population of asthmatic patients with non-reversible bronchial obstruction according to the criteria of the ATS/ERS showed a positive response based on alternative criteria, seen in Fig. 2. Hence, 47 patients (94%) of the total of 50 patients with positive bronchodilator response by the ATS/ERS criteria also had reversible bronchial obstruction by the new criteria. On the other hand, 36 patients (72%) of the total 50 patients with negative bronchodilator response were reclassified as positive bronchodilators according to at least one of the alternative new criteria. The percentage change of sGaw was the most responsible for detecting bronchodilatation, which was positive in 22 patients (44%), followed by the percentage change of PEF in 17 patients (34%) and the absolute change of FEF25–75% in 16 patients (32%). Finally, 10 patients (20%) had a positive absolute change of PEF while 6 patients (12%) had a positive percentage change of FEF25–75%.
Alternative lung function criteria for assessing bronchodilatation in a population of asthmatic patients classified as negative bronchodilators according to ATS/ERS criteria. Patients without bronchodilatation according to ATS/ERS criteria (n=50) were considered as positive bronchodilators according to new lung function criteria. A total of 36 patients (72%) had at least one positive criteria; 22 patients (44%) had a percentage change of sGaw ≥25%; 17 patients (34%) had a percentage change of PEF ≥8%; 16 patients (32%) had an absolute change of FEF25–75% ≥0.087L/s; 10 patients (20%) had an absolute change of PEF ≥0.4L/s; and 6 patients (12%) had a percentage change of FEF25–75% ≥27%. PEF: peak expiratory flow; FEF25–75%: maximum mid-forced expiratory flow; IC: inspiratory capacity; Raw: airway resistance; sGaw: specific airway conductance.
Asthma is a worldwide disease with an increasing incidence and significant burden. Although the diagnosis of asthma is primarily based on clinical grounds, measurement of pulmonary function and particularly the assessment of bronchodilator response is essential for the confirmation and assessment of the disease.1,2
International guidelines define significant bronchodilator response as absolute and percentage changes in FEV1 and FVC, but these variables may be insufficient to identify all the asthmatic patients who exhibit some degree of airway obstruction reversibility.22 Therefore, we aimed to determine the bronchodilator effects on other lung function parameters besides FEV1 and FVC, and its ability to detect functional responses to bronchodilator in asthmatic patients.
The postbronchodilator percentage variation of PEF, FEF25–75% and sGaw had a significant value of AUC on ROC curves, which confirms its usefulness as alternative criteria of bronchodilator positivity. The same was also verified for the absolute change of PEF and FEF25–75% but not for absolute change of sGaw. The absolute and percentage changes in both RV and Raw did not prove to be good enough tests to assess bronchodilator response.
Based on the cut-off values defined by the ROC curves, it was possible to show that a sizeable proportion (72%) of the asthmatic patients without reversibility of bronchial obstruction according to the criteria of the ATS/ERS 2005 guidelines had a positive response based on alternative criteria. The percentage change of sGaw was the most sensitive test for assessing bronchodilatation, followed by the absolute and percentage change of PEF. The absolute and percentage change of FEF25–75% were the least sensitive tests.
Some of our findings do not entirely correlate with previous studies published by Light et al.23 concerning Raw and sGaw. Despite the fact that Raw and sGaw did not add significant information in that study, these parameters did show a higher sensitivity than FEV1 and FVC to predict bronchodilator responses. Nevertheless, more recent studies suggest that these parameters can in fact accurately predict FEV1 reversibility, but these have been carried out mostly among children and adolescent populations.16,24 However, one particular study showed that sGaw and FEF25–75% were the most sensitive pulmonary function measurements to detect bronchodilatation among mild asthmatic patients but these results could not be confirmed in patients with moderate disease.25 Conversely, in our study, both these parameters, particularly sGaw, were able to demonstrate bronchodilator reversibility despite the fact that most of our patients had moderate asthma (FEV1 <80% of predicted).
Regarding PEF, other authors also reported that reversibility of airflow obstruction in patients with obstructive lung diseases could be shown by PEF measurements in a comparable way to those obtained by FEV1.15
In our study, FEF25–75% significantly contributed to the assessment of reversibility of airways in asthmatic patients. However, caution should be taken when considering FEF25–75% alone; it only should be contemplated when accompanied by an improvement in FEV1 and/or FVC, and also, the measurement should be done isovolumetrically.
Our study has a number of strengths and limitations. All patients were never-smokers diagnosed with asthma based on clinical grounds; they probably were representative of a typical population of asthmatic patients. Both groups were demographically homogeneous thus avoiding possible bias. Nevertheless, our cohort is number limited and larger samples would be necessary to draw additional conclusions. Another important limitation is the lack of a control group represented by normal subjects without asthma.
ConclusionsAssessment of reversibility is part of the common evaluation of patients with asthma or other obstructive pulmonary diseases, however international criteria may underestimate significant functional responses to bronchodilators in asthmatic patients. Based on our results, attention should be given to other functional parameters but the mainstream of bronchodilator treatment should not obviate the symptomatic improvements reported by each patient.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.