Screening methods have become increasingly important owing to the growing number of patients suspected of obstructive sleep apnea (OSA) being referred for sleep consultation. The STOP-Bang questionnaire has been validated as a screening tool for OSA in surgical patients.
ObjectivesTo evaluate the performance of a Portuguese version of the STOP-Bang questionnaire for the diagnosis of OSA in a sleep clinic.
MethodsProspectively, for 2 months, all patients referred to our clinic for clinical evaluation completed a translated version of the STOP-Bang questionnaire in Portuguese and underwent a sleep study.
ResultsWe observed 216 patients and 215 (99.5%) were included. Age was 53.63±13.10 years, 63.3% were male patients, neck circumference was 40.4±44.11cm and BMI was 29.41 [26.85; 33.06]kg/m2. OSA was present in 78% of the patients, of whom, 33% had moderate and 37% had severe OSA. A STOP-Bang score ≥3 had a sensitivity and positive predictive value (PPV) for OSA of 93.4% and 86.6%, respectively. Each increase in the STOP-Bang score was associated with an increase in the probability of OSA and severe OSA; reaching a 95% OSA probability, for a score of 6, and a 73% severe OSA probability, for a score of 8. A score of 3 and 2 had a negative predictive value for moderate/severe OSA of 85.3% and 91.7%, respectively.
ConclusionsThe STOP-Bang questionnaire showed high sensitivity and PPV for OSA with the probability of severe OSA steadily increasing, the higher the scores. Furthermore, a low score showed high predictive value for the exclusion of moderate/severe OSA. The STOP-Bang questionnaire can be a powerful tool for stratifying patients in the diagnosis of OSA.
Obstructive sleep apnea (OSA) is a highly prevalent disorder affecting up to 20% of the general population1 and can occur in all age groups.2 OSA, even if asymptomatic, is independently associated with increased morbidity and mortality due to cardiovascular and neurovascular diseases, metabolic disorders, and impaired neurocognitive function.3–5 Although prevalent, it has been estimated that 82% of men and 92% of women with moderate/severe OSA may be undiagnosed.6
Consequently a simple and reliable method of screening certain populations, namely high-risk groups, is needed. The choice of screening method will depend on its ability to achieve a specific objective: to include patients with OSA for appropriate sleep testing; to detect those with more severe disease prompting diagnosis and treatment; and to exclude patients without OSA or those without moderate/severe OSA whose evaluation and treatment is less pressing. Such screening methods are increasingly important due to the growing number of suspected OSA patients being referred to sleep clinics. Numerous clinical prediction models have been developed based upon self-reported symptoms, demographics, anthropometric variables, and comorbidities.7,8 One tool commonly used to identify patients with potential sleep disorders is the Epworth Sleepiness Scale (ESS), but the ESS was developed to measure propensity for sleep onset rather than the likelihood of sleep disordered breathing.9,10 A recently proposed screening method is the STOP-Bang questionnaire,11 a scoring model consisting of eight Yes/No questions (score: 1/0, with scores ranging from a value of 0 to 8), which make it a potentially simple and easy to use screening method. A score of ≥3 has shown a high sensitivity (83.6%) for detecting OSA in a surgical population, but also in detecting moderate and severe OSA (92.9% and 100%, respectively).11
We assume that increasing the number of risk factors for OSA, as reflected in the STOP-Bang questionnaire, increases the probability of having OSA but also increases the likelihood of having other severe diseases. We hypothesize that using the STOP-Bang questionnaire in a sleep medicine clinic may show a correlation between the model and the severity of OSA, allowing us to determine a set of predicted probabilities for different disease severities. This could ultimately enable clinicians, in sleep medicine clinics, to make more reasoned decisions about inclusion, exclusion or the prioritization of patients for diagnostic and therapeutic evaluations.
MethodsSTOP-Bang questionnaire translationThe Portuguese version of the STOP-Bang questionnaire was obtained using the following stages: translation; back translation; comparison of the back translation with the original English version by a committee; and usage with bilingual individuals. Two independent translators translated the original questionnaire11 into Portuguese and two other independent translators did the back translations. A committee whose members were fluent in English fused the back-translated versions into a single one and compared it to the original English version. Every individual question was analyzed according to the methods described.12 The committee made the necessary adjustments and approved a final Portuguese version (Annex 1). In order to evaluate the equivalence between the original English and the Portuguese version, 13 bilingual individuals completed both versions, first the original version and, after a week, the Portuguese version. Questions relating to the STOP portion were read and answered by the individuals, and age, gender, weight, height and neck circumference were measured and recorded in order to fill the Bang portion of the questionnaire. Correlations between the scores obtained by both versions were calculated.
Subjects and designDuring a two-month period, from December 2012, patients referred to the clinic, aged 18 years or older, were asked if they were willing to be included in the study, independently of the reasons for being referred, which included referral for isolated symptoms, clinical suspicion of a specific sleep disorder (e.g. insomnia, parasomnia or OSA) or for screening of a sleep breathing disorder due to a recent cardiovascular event. During the clinical consultation, all the patients were asked to complete the STOP questionnaire,11 and information concerning body mass index (BMI), age, neck circumference, and gender (Bang)11 was collected by a clinician/research assistant. The research staff gave no help in the interpretation or answering of the questionnaires. A Sleep Disorders Questionnaire and ESS were previously mailed and answered at home. In the following two months, all the patients were invited to undergo a portable or laboratory polysomnography (PSG). Patients previously diagnosed with OSA or unable to read or write were not included in the study.
Sleep studies, scoring and diagnosisA laboratory PSG was performed when patients had a low clinical probability of OSA (as judged by the physician) or if there was a clinical suspicion that other sleep disorders might be present (isolated or associated with OSA). Patients with a moderate to high clinical probability were evaluated with a portable PSG. In cases of technical errors or if the clinical context was not explained by a negative portable PSG a laboratory PSG was ultimately performed.
Portable PSG was performed with a level III equipment (Alice PDx, Embletta X100, Nox T3 or Stardust II) that has been shown to be a reliable alternative to standard PSG in the diagnosis of OSA in adult patients.13–15 PSG recordings were carried out in the patients’ homes. Recordings included nasal airflow, thoracic and abdominal respiratory effort, pulse oximeter, body position sensor, and lower limb electromyography (EMG) (when available on the device). The patients and their aides were instructed, by a trained PSG technician, on how to connect and remove the device. The overnight home recordings were unattended. The following morning the patient removed the device and returned it to the sleep clinic. A sleep technician manually scored the recordings and a physician informed the patient of the results. Laboratory PSG was performed overnight and patients went to bed at their usual bedtime. A standard montage obtained with surface electrodes was used consisting of: electroencephalogram, electrooculogram, submental and lower limb EMG, and electrocardiogram. Additional recordings included: oronasal airflow (thermistor and pressure sensor), thoracic and abdominal respiratory effort, pulse oximeter, snore and body position sensor. A well-trained PSG technician scored the PSG recordings under the supervision of a sleep physician who assessed and approved the reports. The technician and sleep physician were blind to the study information (STOP-Bang score and clinical information). Manual scoring was performed according to the Manual of the American Academy of Sleep Medicine,16 and the American Academy of Sleep Medicine Task Force recommendations.17 The diagnosis and severity of OSA were classified based on the Apnea-Hypopnea Index values: >5–15/h as mild, >15–30/h as moderate, and >30/h as severe.16,17 Other diagnoses, in accordance with the International Classification of Sleep Disorders,18 were based on: clinical characteristics; minimal follow-up of at least 6 months; and results from laboratory PSG, multiple sleep latency test, or actigraphy.
AnalysisStatistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 20 and MedCalc version 12. Patients’ characteristics are presented with the following descriptive statistics: mean and standard deviation were used for normally distributed continuous data; median and inter-quartile range were used for non-normally distributed continuous data; and frequency and percentage were used for categorical data. Reproducibility between the original and Portuguese version was assessed calculating the correlation of the scores from both versions with Pearson's correlation and using Cronbach's alpha to obtain intraclass correlation. OSA and non-OSA groups were compared with Student's t-test for means, Mann–Whitney U test for medians and Chi-square test for proportions. To assess the performance of the STOP-Bang questionnaire and the Epworth Sleepiness Scale in predicting OSA, sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs) were calculated for each score and for different AHI cut-offs. Area under the receiver-operating curve (ROC) was also calculated to assess the diagnostic ability of the STOP-Bang questionnaire and the Epworth Sleepiness Scale for different AHI cut-offs. Logistic regression was used to compare the severity of the AHI with the STOP-Bang score and to calculate predicted probabilities for different AHI cut-offs at each score.
ResultsIn the group of bilingual individuals, the mean scores on the original STOP-Bang and Portuguese STOP-Bang were 2.4±1.7 and 2.3±1.7, respectively, showing good reproducibility between the original and the translated questionnaire (intraclass correlation coefficient=0.932; Pearson's r=0.928; p<0.0001).
A total of 216 patients were observed during the study period, of which 215 (99.5%) completed the STOP-Bang questionnaire and performed a portable or laboratory PSG. Of the 215 patients included in the final analysis, 137 (63.7%) performed a portable PSG and 78 (36.2%) performed a laboratory PSG. Of these 78 patients, 19 had previously performed a portable PSG, but due to technical errors or a clinical context the condition was not explained by a normal or near normal portable PSG, a laboratory PSG was ultimately performed. There were 6 patients in whom a negative portable PSG was not followed by a laboratory PSG because the study had been performed in excellent technical conditions and the patients described a full night's sleep.
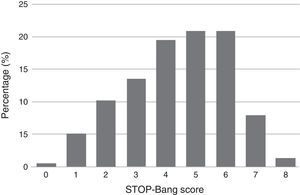
Descriptive summary statistics, STOP-Bang characteristics, AHI of the patient population and comparison of OSA and non-OSA groups are displayed in Table 1. Although greater or more prevalent in the OSA group, Epworth score, tiredness, BMI >35kg/m2, and age >50 years were the only characteristics which were not significantly different between the OSA and non-OSA group. Of the 215 patients included for analysis 168 (78%) had OSA of which, 50 (29.8%), 56 (33.3%), and 62 (36.9%) had mild, moderate, and severe OSA, respectively. In the non-OSA group, 3.4% of the patients had no sleep disorder while 9.3% had isolated insomnia, 3.3% had isolated restless legs, 2.3% had central sleep apnea syndromes, 1.7% had idiopathic hypersomnia, 1.0% had narcolepsy, 1.0% had parasomnia and 0.5% had period leg movement disorder (isolated). The distribution of patients according to their STOP-Bang score is detailed in Fig. 1. Most patients had a STOP-Bang score of 4 (19.5%), 5 or 6 (both with 20.9%).
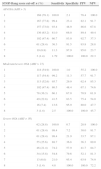
Summary statistics of the patient population and comparison of OSA and non OSA group.
| All | OSA (AHI>5) | Non-OSA (AHI<5) | p (OSA vs. nOSA) | |
|---|---|---|---|---|
| n (%) | 215 | 168 (78%) | 47 (22%) | |
| Age (years) | 53.63±13.10 | 55.14±12.98 | 48.23±12.18 | 0.0001 |
| BMI (kg/m2) | 29.41 [26.85; 33.06] | 30.11 [27.69; 34.13] | 26.91 [25.40; 29.39] | <0.0001 |
| Neck circumference (cm) | 40.44±4.11 | 41.32±3.75 | 37.31±3.83 | <0.0001 |
| Epworth's score | 10.00 [5; 15] | 10 [5; 16] | 8 [4; 14] | 0.237 (NS) |
| Snore (%) | 73.0 | 81.5 | 42.6 | <0.0001 |
| Tiredness (%) | 61.9 | 63.7 | 55.3 | 0.296 (NS) |
| Observed apnea (%) | 52.6 | 58.3 | 31.9 | 0.0001 |
| Pressure (high blood) (%) | 57.2 | 63.1 | 36.2 | 0.0001 |
| BMI>35kg/m2 (%) | 16.3 | 17.9 | 10.6 | 0.236 (NS) |
| Age>50 years (%) | 60.5 | 62.5 | 53.2 | 0.249 (NS) |
| Neck circ.>40cm (%) | 56.3 | 65.5 | 23.4 | <0.0001 |
| Gender (male) (%) | 63.3 | 71.4 | 34.0 | <0.0001 |
| STOP-Bang score | 4.41±1.70 | 4.84±1.45 | 2.87±1.66 | <0.0001 |
| AHI (h) | 16.7 [7.8; 32.9] | 21.75 [13.52; 38.92] | 1.6 [0.4; 4.0] | <0.0001 |
BMI: body mass index; AHI: Apnea-Hypopnea Index; OSA: obstructive sleep apnea; NS: not significant.
Using the STOP-Bang model for the prediction of all OSA, moderate/severe OSA, and severe OSA, the area under the ROC was 0.806 (95% CI: 0.730–0.881) (p<0.0001), 0.730 (95% CI: 0.661–0.798) (p<0.0001), and 0.728 (95% CI: 0.655–0.801) (p<0.0001), respectively, confirming the diagnostic ability of the STOP-Bang model in all OSA severities. Using the ESS as predictor, the area under the ROC was 0.556 (95% CI: 0.461–0.652) (p=0.238), 0.606 (95% CI: 0.529–0.682) (p=0.008), and 0.614 (95% CI: 0.533–0.696) (p=0.009), for all OSA, moderate/severe OSA, and severe OSA, respectively, reflecting the inferior discriminative power of the ESS for the diagnosis of OSA.
The sensitivity, specificity, PPV, and NPV for all OSA, moderate/severe OSA, and severe OSA are summarized in Table 2. With a STOP-Bang score ≥3 the sensitivity and PPV were 93.4% and 86.6% for all OSA, 95.7% and 62.4% for moderate/severe OSA and 98.4% and 33.7% for severe OSA, respectively. With the same cut-off the negative predictive value for moderate/severe and severe OSA were 85.3% and 97.1%, respectively. As the STOP-Bang score increased from 3 to 7, the specificity and PPV increased continuously from 48.9% to 97.9% and 86.6% to 95.0% for all OSA; 29.9% to 95.5% and 62.4% to 80% for moderate/severe OSA; and 21.9% to 95.4% and 33.7% to 63.9% for severe OSA, respectively.
Predictive parameters of each STOP-Bang score cut-offs for different AHI levels (n, number of patients in the AHI group who scored the STOP-Bang score indicated or higher; percentage out of the 215 patients).
| STOP-Bang score cut-off | n (%) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| All OSA (AHI>5) | |||||
| 1 | 168 (78.1) | 100.0 | 2.1 | 78.4 | 100.0 |
| 2 | 167 (77.8) | 99.4 | 23.4 | 82.1 | 91.7 |
| 3 | 157 (73.0) | 93.4 | 48.9 | 86.6 | 67.8 |
| 4 | 136 (63.2) | 81.0 | 66.0 | 89.4 | 49.4 |
| 5 | 102 (47.4) | 60.7 | 83.0 | 92.7 | 37.3 |
| 6 | 61 (28.4) | 36.3 | 91.5 | 93.8 | 28.8 |
| 7 | 19 (8.8) | 11.3 | 97.9 | 95.0 | 23.7 |
| 8 | 3 (1.4) | 1.79 | 100.0 | 100.0 | 22.3 |
| Moderate/severe OSA (AHI>15) | |||||
| 1 | 118 (54.9) | 100.0 | 1.0 | 55.2 | 100.0 |
| 2 | 117 (54.4) | 99.2 | 11.3 | 57.7 | 91.7 |
| 3 | 113 (52.6) | 95.7 | 29.9 | 62.4 | 85.3 |
| 4 | 102 (47.4) | 86.5 | 48.4 | 67.1 | 74.6 |
| 5 | 78 (36.3) | 66.1 | 67.0 | 70.9 | 61.9 |
| 6 | 49 (22.8) | 41.5 | 83.5 | 75.4 | 54.0 |
| 7 | 16 (7.4) | 13.6 | 95.9 | 80.0 | 47.7 |
| 8 | 3 (1.4) | 2.5 | 100.0 | 100.0 | 45.7 |
| Severe OSA (AHI>30) | |||||
| 1 | 62 (28.8) | 100.0 | 0.7 | 28.9 | 100.0 |
| 2 | 61 (28.4) | 98.4 | 7.2 | 30.0 | 91.7 |
| 3 | 61 (28.4) | 98.4 | 21.9 | 33.7 | 97.1 |
| 4 | 55 (25.6) | 88.7 | 36.8 | 36.3 | 89.0 |
| 5 | 46 (21.4) | 74.2 | 57.9 | 41.7 | 84.7 |
| 6 | 34 (15.8) | 54.8 | 79.6 | 52.1 | 81.3 |
| 7 | 13 (6.0) | 21.0 | 95.4 | 63.9 | 74.9 |
| 8 | 3 (1.4) | 4.8 | 100.0 | 100.0 | 72.2 |
AHI: Apnea-Hypopnea Index; OSA: obstructive sleep apnea; PPV: positive predictive value; NPV: negative predictive value.
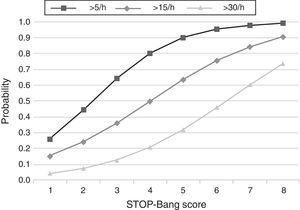
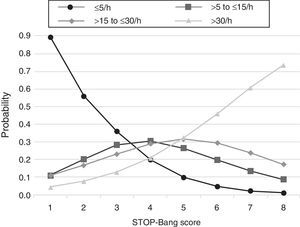
The predicted probabilities of having OSA, moderate/severe OSA, and severe OSA are shown in Table 3. As the STOP-Bang score increased from 2 to 8, the probability of having OSA, moderate/severe OSA, and severe OSA increased continuously from 44% to 99%, 24% to 90% and 7% to 73%, respectively. This trend, present in all groups is illustrated in Fig. 2. The predicted probabilities of having OSA of a specific severity are illustrated in Fig. 3. With each incremental increase in the score from 0 to 4, the probability of having no sleep apnea diminished, while the probability of having mild, moderate, or severe sleep apnea increased. With any score greater than 4, only the probability of having severe sleep apnea increased continuously.
Predicted probabilities of each STOP-Bang score for different AHI levels (n, number of patients in the AHI group who scored the STOP-Bang score indicated; percentage out of the 215 patients).
| Score | All OSA (AHI>5) | Mod./severe OSA (AHI>15) | Severe OSA (AHI>30) | |||
|---|---|---|---|---|---|---|
| n (%) | Probability | n (%) | Probability | n (%) | Probability | |
| 1 | 1 (0.6) | 0.26 | 1 (0.8) | 0.15 | 1 (1.6) | 0.04 |
| 2 | 10 (6.0) | 0.44 | 4 (3.4) | 0.24 | 0 (0.0) | 0.07 |
| 3 | 21 (12.5) | 0.64 | 11 (9.3) | 0.36 | 6 (9.7) | 0.13 |
| 4 | 34 (20.2) | 0.80 | 24 (20.3) | 0.50 | 9 (14.5) | 0.21 |
| 5 | 41 (24.2) | 0.90 | 29 (24.6) | 0.64 | 12 (19.4) | 0.32 |
| 6 | 42 (25.0) | 0.95 | 33 (28.0) | 0.75 | 21 (33.9) | 0.46 |
| 7 | 16 (9.5) | 0.98 | 13 (11.0) | 0.84 | 10 (16.1) | 0.61 |
| 8 | 3 (1.8) | 0.99 | 3 (2.5) | 0.90 | 3 (4.8) | 0.73 |
AHI: Apnea-Hypopnea Index; OSA: obstructive sleep apnea.
This study shows that, in a population referred to a sleep medicine clinic, a STOP-Bang score ≥3 has high sensitivity (93.4%) and PPV (86.6%) for the diagnosis of OSA and that the greater the STOP-Bang score, reflecting a higher cumulative score of known risk factors, the greater the probability of sleep apnea, particularly severe sleep apnea (Fig. 3). The probability of OSA for score of 3 is of 64% and increases continuously to 80%, 90% and 95% with a stepwise increase of the STOP-Bang score to 4, 5, and 6. This performance is due to the continuously increasing probability of severe OSA for each score above 4, reaching a predicted probability of 46%, 61% and 73% for a score of 6, 7 and 8 (Fig. 3), respectively. Moreover, a score lower than 3 showed high discriminative power to exclude moderate to severe OSA, as reflected by a negative predictive value of 85.3% and 91.7% for a score of 3 and 2, respectively. The area under the ROC curve was consistently high for the diagnostic ability of the STOP-Bang questionnaire for all OSA severities. On the contrary, the ESS, as expected, showed inferior ability for the prediction of moderate to severe and severe OSA and was non-significant for the prediction of all OSA. Furthermore, the ESS and tiredness (as assessed by question T of the STOP-Bang questionnaire) were statistically similar between the OSA and non-OSA groups, albeit tending to be greater and more prevalent in the OSA group, reflecting excessive daytime sleepiness and tiredness as similarly common complaints among patients with various sleep disorders. Two other STOP-Bang characteristics, BMI>35kg/m2 and age >50 years, were also statistically not different, although tending to be more prevalent in the OSA group. This might be related to the specific cut-off values of the questionnaire, as the OSA group was statistically more obese and older.
Due to the relatively high prevalence of undiagnosed OSA and its short and long-term complications, a reliable screening tool is required for a quick prediction of OSA. A fast and reliable screening test can enable clinicians within a given clinical context to make more reasoned diagnostic decisions, namely in stratifying patients for unrecognized OSA and triage for further diagnostic assessment and/or treatment. Questionnaires can be appropriate tools for that purpose since they can be applied and scored easily as part of routine daily practice. Furthermore, analysis of the questionnaire's performance in specific populations can provide clinicians with a set of predictive parameters for various levels of OSA severity, which can be used as a valuable guide for diagnostic or therapeutic decisions. In our study the present results show that the STOP-Bang questionnaire, in the context of a sleep medicine clinic, can be a very useful tool for triaging patients into three groups depending on their score: score of 0–2 – low probability of OSA and very low probability of moderate/severe OSA; score of 3–4 – probable OSA; score of 5–8 – high probability of moderate/severe OSA.
Several questionnaires and other clinical screening tests for obstructive sleep apnea have been analyzed. In the meta-analysis by Ramachandran and Josephs19 that included the American Society of Anesthesiologists (ASA) checklist,20 the Berlin questionnaire,21 the Sleep Questionnaire,22 the Sleep Disorders Questionnaire (SDQ),23 and the STOP and STOP-Bang questionnaires,11 the authors concluded that the Berlin questionnaire and the SDQ were the most accurate questionnaires overall to screen OSA, while the ESS was the least accurate. The authors identified the STOP-Bang questionnaire as an inferior screening tool but an excellent method for predicting severe OSA due to its simplicity and relative ease of use. Another systematic review, by Abrishami et al.24 reported that the Wisconsin25 and the Berlin questionnaires had the highest sensitivity and specificity, respectively. However, the authors argue that the validity of the studies was unclear due to potential selection bias as subjects in the Berlin study were pre-screened for the presence of snoring, wake-time sleepiness or fatigue, and history of obesity or hypertension. In terms of predicting moderate or severe OSA, the authors concluded that the STOP-Bang and the Berlin questionnaires were found to have the highest sensitivity and specificity, respectively. They also noted that the Berlin questionnaire, which was shown to have high sensitivity for detecting OSA (69–86%), was found to be relatively less sensitive in detecting moderate and severe cases, while the STOP-Bang questionnaire was shown to have consistently high sensitivity for detecting OSA at different severity levels. For these reasons, Abrishami et al.24 and Ramachandran and Josephs19 recommended the STOP-Bang questionnaire for OSA screening due to its high-quality methodology and reasonably accurate results. However, in both reviews there were discrepancies between the analyzed populations and there was only one study included that evaluated the STOP-Bang questionnaire in surgical patients.11
Recently, there have been two studies of the STOP-Bang questionnaire performance in patients referred to a sleep medicine clinic,26,27 and an additional study by Chung et al.28 in a surgical population. Farney et al.26 showed an 85.1% probability of OSA for a score of 3 or greater. This was consistent with the papers by Chung et al.,11,28 in surgical populations, which reported a 75.3% probability and 84% sensitivity for OSA with a score of 3 or greater. For the same score, we show better results as reflected by the 86.6% probability and 93.4% sensitivity for OSA. Compared to the study by Chung et al.,28 our better results could be related with the greater prevalence of OSA in our population (78% vs. 68.4%) and by a greater proportion of moderate/severe OSA patients (70.0% vs. 56.3%). Also, Farney et al.26 reported, as we do, that with any score greater than 4, the probability of severe sleep apnea increases continuously. Silva et al.27 compared the performance of the STOP-Bang questionnaire, in a sleep medicine clinic, with the STOP questionnaire,11 the ESS,9 and a 4-variable screening tool.29 They concluded that the STOP-Bang tool identified more subjects with moderate-to-severe OSA and severe OSA while the ESS was the least capable tool for this purpose as it focuses on sleep propensity rather than OSA. For a score cut-off of 3, the reported sensitivities were of 87% and 70.4% for moderate to severe and severe OSA, respectively. Chung et al.11,28 showed, for that cut-off, similar sensitivities for moderate to severe OSA (68.4% and 92.9%) but better results for severe OSA (94.8% and 100%). In our study, the corresponding sensitivities were of 95.7% and 98.4% for moderate to severe and severe OSA, respectively. Our results and those described before confirm the high performance of the STOP-Bang for diagnosing OSA but especially moderate to severe and severe OSA.
The studies by Farney et al.26 and Silva et al.27 were cross-sectional analysis of patients who had undergone diagnostic PSG for several reasons and whose STOP-Bang responses were retrospectively derived from answers to other questionnaires that the authors defined as similar to those in the STOP-Bang questionnaire. Our cross-sectional analysis has some advantages here, as it was designed specifically for the present analysis, conducted prospectively and had a high rate of patients with both a complete STOP-Bang questionnaire and sleep study (99.5%). Furthermore, during the study, the technicians and clinical personnel validating the sleep studies were blind to the results of the STOP-Bang questionnaire and there was only a short waiting time between the answering of the questionnaires and the execution of the sleep study. There are some potential disadvantages, as our study has a smaller population compared to the studies of Farney et al. and Silva et al., and includes results from both laboratory and portable (level III) PSG. The use of level III devices could mean some underestimation in the measured AHI. Also, 6 patients (2.8% of the total) had a negative portable PSG, which was not confirmed by performing a laboratory PSG. This was a small percentage of patients and, although unlikely, it is possible that a false positive could have occurred. Moreover, only those performed in excellent technical conditions and with a patients’ description of a full night of sleep were not reevaluated and, as previously stated, level III portable sleep devices have been shown to be a reliable alternative to a standard PSG in the diagnosis of OSA in adult patients.13–15
We conclude that the STOP-Bang questionnaire can be a powerful tool in the assessment of patients referred to a sleep medicine clinic as it shows a high level of performance in the diagnosis of OSA and detection of moderate to severe OSA, but also in the exclusion of moderate to severe disease. These characteristics may help to define the STOP-Bang questionnaire as an interesting triage tool in the context of a sleep medicine clinic as it can: include patients with probable OSA for diagnostic evaluation; predict those with more severe disease, who are in greater need of therapy and may be more quickly diagnosed with simpler sleep studies; and virtually exclude patients from a moderate/severe OSA diagnosis.
FundingThis study received no industry support. The authors have no financial conflicts of interest to report.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.