Sarcoidosis is a systemic granulomatous disease of unknown etiology. Epidemiological studies of different populations are essential because clinical presentation, organ involvement, disease severity, and prognosis vary significantly according to region and population. The aim of this study was to assess epidemiological and clinical characteristics, staging factors, and clinical course in patients with sarcoidosis from a tertiary hospital in Oporto, Portugal.
MethodsA retrospective analysis of patients with sarcoidosis and at least 2 years of follow-up evaluated at the Centro Hospitalar de São João between 2000 and 2014.
ResultsWe identified 409 patients with sarcoidosis (females, 58.9%; mean age at diagnosis, 38.9±13.4 years; smokers, 14.4%]. All the patients were diagnosed according to the ERS/ATS/WASOG consensus statement and 64.1% had evidence of noncaseating epithelioid cell granulomas in biopsy specimens. Bronchoalveolar lavage was performed as part of the diagnostic work-up in 289 patients and 90.2% had lymphocytosis (CD4/CD8 ratio ≥3.5 in 60.9% of cases). Exertion dyspnea, cough, and constitutional symptoms were the most common presenting symptoms; 10.1% of patients were asymptomatic, 22.8% had Löfgren syndrome, and 50.5% had extrathoracic involvement. Radiographic stages of disease according to the Scadding criteria were as follows: stage 0 (5.2%), stage I (33.7%), stage II (47.0%), stage III (8.4%), and stage IV (5.7%). Impaired respiratory function was observed in 45.6% patients and was mostly mild. Systemic treatment was administered in 58.6% of cases. Overall, 45.3% of patients experienced disease resolution.
ConclusionThe epidemiological and clinical characteristics of this cohort of patients with sarcoidosis from the Oporto region in northern Portugal revealed epidemiological and clinical characteristics that were generally similar to those described in other Western Europe populations and in the US ACCESS study. However, we found a higher proportion of patients who progressed to chronic forms.
Sarcoidosis is a multisystem inflammatory disorder of unknown etiology characterized by the accumulation of macrophages and CD4+ T lymphocytes in involved organs. It is associated with the formation of noncaseating granulomas and mainly affects the lungs, lymph nodes, and skin.1,2 The prevailing hypothesis regarding the pathogenesis of sarcoidosis is that it is caused by various unknown antigens in genetically susceptible hosts and that these factors modulate both incidence and clinical phenotype.3,4
Sarcoidosis is diagnosed by clinical findings, chest radiographic or high-resolution computed tomography features, and a biopsy showing noncaseating granulomas.2,5–7 Bronchoalveolar lavage (BAL), which is a standard procedure in the diagnostic work-up of patients with diffuse lung disease, has also proven very useful, since a high CD4/CD8 ratio (>3.5) is considered to be highly specific (93–96%) for sarcoidosis and, when found in association with a typical clinical picture, may support the diagnosis and obviate the need for confirmation by additional procedures.8–10
The prevalence of sarcoidosis is estimated at between 15.3 and 21.7 cases per 100,000 population depending on the series and geographical location.11,12 The female to male ratio is between 1.2 and 1.5/1.12–15 About 70% of patients are 25–40 years old at the time of presentation.13,14,16 In some multiethnic populations, sarcoidosis is more prevalent in black people. In the USA, for example, it is four times more common in blacks than in whites.11
Clinical presentation, organ involvement, disease severity, and clinical course also vary significantly according to region and population.11–13,15,16 It is therefore of the utmost importance to accurately define the characteristics of the disease in each region in order to tailor diagnostic and treatment approaches appropriately. The purpose of the present study was to assess epidemiological characteristics, clinical presentation, staging factors, and clinical course in patients with sarcoidosis evaluated at a tertiary hospital in the north of Portugal.
Materials and methodsThis study was designed and conducted by the Diffuse Lung Disease study group at the Centro Hospitalar de São João (CHSJ) in Oporto, Portugal. The CHSJ is a tertiary hospital that receives patients mostly from the Oporto district and the north of Portugal. We performed a retrospective analysis of patients with a diagnosis of sarcoidosis and at least 2 years of follow-up seen at the CHSJ outpatient clinic between 2000 and 2014.
All the patients had been diagnosed in accordance with the consensus statement of the American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Disorders (ATS/ERS//WASOG).4 The diagnosis was based on compatible clinical and radiological findings, supported by histological evidence of noncaseating granulomatous inflammation. The diagnosis was also assumed, even without histological confirmation, in patients with compatible clinical and radiographic features, BAL fluid CD4/CD8 ratio >3.5, and at least 2-year observation period to exclude other medical conditions. BAL was performed according to ERS recommendations, with appropriate processing and interpretation of specimens.17
Löfgren syndrome was defined as erythema nodosum combined with bilateral hilar lymphadenopathy on chest radiograph and frequent fever and ankle arthralgia.6 Thoracic involvement at the time of diagnosis was classified using the Scadding radiological criteria (stage 0, no intrathoracic involvement; stage I, bilateral hilar lymphadenopathy without lung involvement; stage II, bilateral hilar lymphadenopathy with lung involvement; stage III, lung involvement only; and stage IV, lung fibrosis).6
Additionally, the patients underwent lung function tests and ventilation patterns were classified as normal, obstructive, restrictive, or mixed according to the ERS/ATS Task Force criteria.18
All patients underwent a thorough physical examination to detect extrathoracic involvement, including a meticulous skin evaluation, cardiac auscultation, abdominal palpation, and a neurological examination. Cutaneous lesions suspicious for sarcoidosis were biopsied. The patients also underwent blood (peripheral blood counts, calcium, liver enzymes, creatinine, urea nitrogen, and angiotensin-converting enzyme) and urine analyses. The protocol also included a detailed ophthalmologic evaluation and electrocardiogram and transthoracic echocardiography. When these last exams showed changes or when cardiac symptoms were present, patients were referred for 24-h Holter monitoring and cardiac magnetic resonance (MR).
Extrathoracic involvement was assessed using the WASOG Sarcoidosis Organ Assessment Instrument,19 which classifies organ involvement as “highly probable”, “probable”, or “possible”. An organ was defined as involved with sarcoidosis if there was a “highly probable” or “probable” involvement. In the WASOG instrument the category “definitive organ involvement”, which is used in the ACCESS study (A Case Control Etiologic Study of Sarcoidosis),20,21 was replaced with “highly probable” because even histologic evidence of granulomatous inflammation is not definitive for a diagnosis of sarcoidosis.
In accordance with ATS/ERS//WASOG consensus statement,4 treatment was prescribed whenever patients presented significant symptomatic complaints, meaningful organ impairment and/or sustained disease progression.
Disease resolution was defined as disappearance of symptoms, normalization of chest X-ray findings and lung function, and, in the event of extrathoracic manifestations, regression of clinical, analytical, and/or imaging abnormalities within 2 years of diagnosis. Patients with evidence of disease after 2 years were considered to have chronic sarcoidosis.
Data were obtained through a chart review. The statistical analysis was performed with SPSS for Windows (version 19). Data were presented as mean and standard deviation for continuous variables and percentages for categorical variables. The Student t test for independent samples was used to compare means of normally distributed variables and the Mann–Whitney U rank test was used to compare means of nonnormally distributed variables. P values <0.05 were considered statistically significant.
The study was approved by the ethics committee at the CHSJ.
ResultsA total of 409 patients with a diagnosis of sarcoidosis evaluated between 2000 and 2014 were included in our analysis.
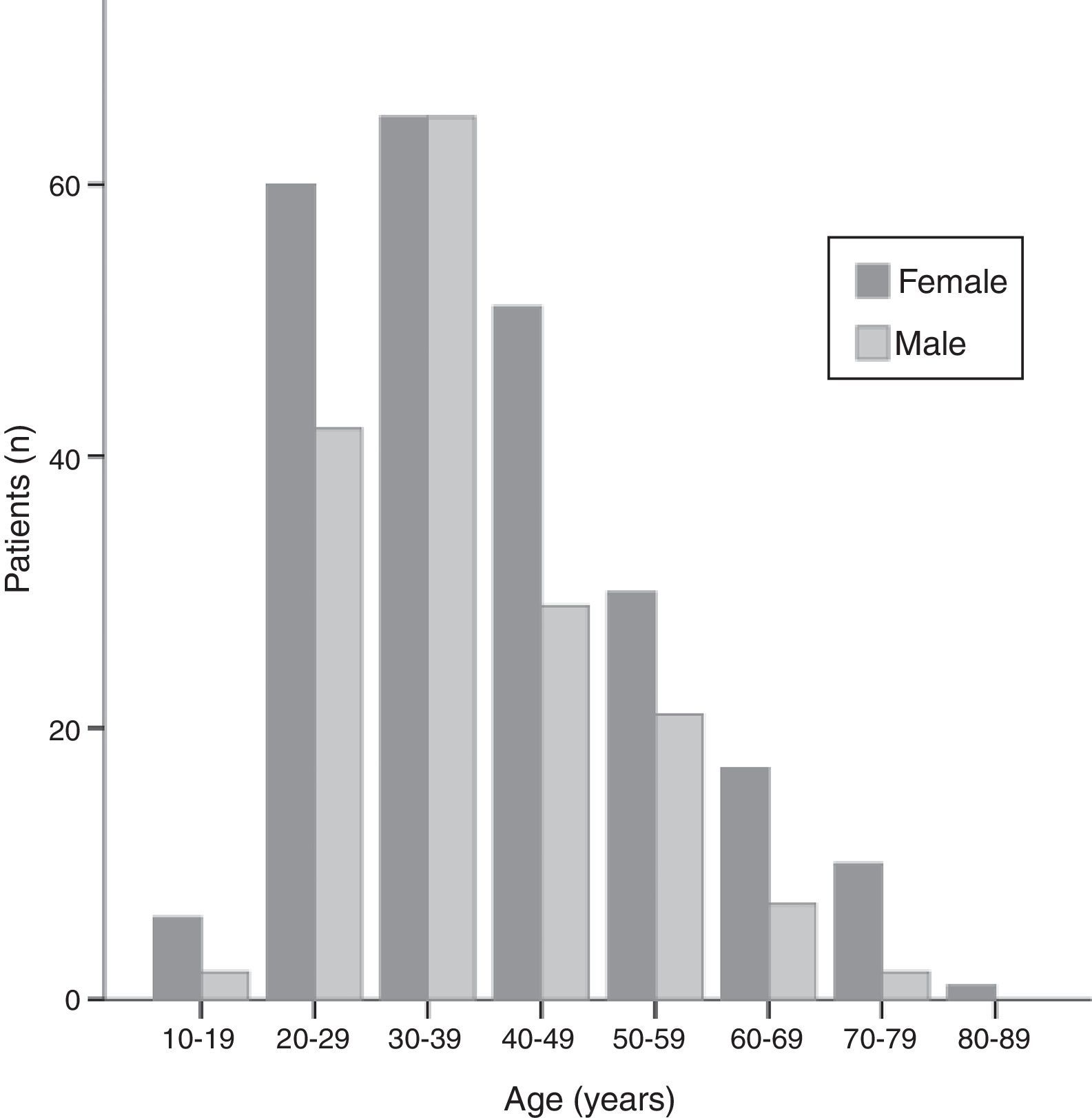
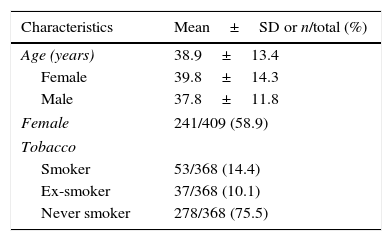
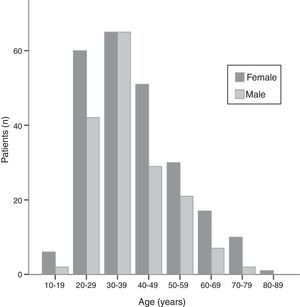
Sociodemographic characteristicsThe mean age at initial diagnosis was 38.9±13.4 years (Fig. 1). There were 241 (58.9%) females and 168 (41.1%) males and their mean age was similar (39.8±14.3 for women vs 37.8±11.8 years for men, P=0.118). The vast majority of patients were white (98.5%); 1.0% were blacks and 0.5% were mixed race. At time of the diagnosis, 14.4% of the patients were smokers and 10.1% were ex-smokers. The patients’ sociodemographic characteristics are summarized in Table 1.
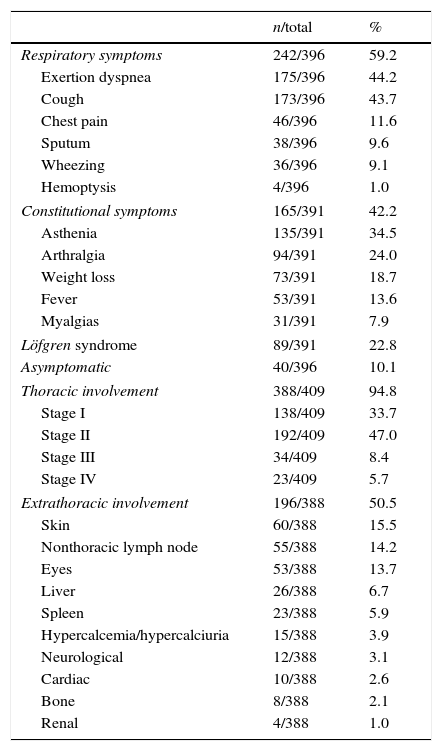
Clinical presentationRespiratory symptoms were mentioned as presenting symptoms by 59.2% of patients; the most common complaints were exertion dyspnea (44.2%) and dry cough (43.7%). Constitutional symptoms were observed in 42.2% of cases, and asthenia was particularly common (34.5%). Löfgren syndrome was the presenting clinical form in 22.8% of patients; 44.7% were female and 12.7% were male (P<0.001). At diagnosis, 10.1% of patients were asymptomatic and the disease was detected in a routine thoracic chest X-ray. Table 2 summarizes the clinical presentations.
Clinical presentations of sarcoidosis (respiratory and constitutional symptoms) and thoracic and extrathoracic involvement.
| n/total | % | |
|---|---|---|
| Respiratory symptoms | 242/396 | 59.2 |
| Exertion dyspnea | 175/396 | 44.2 |
| Cough | 173/396 | 43.7 |
| Chest pain | 46/396 | 11.6 |
| Sputum | 38/396 | 9.6 |
| Wheezing | 36/396 | 9.1 |
| Hemoptysis | 4/396 | 1.0 |
| Constitutional symptoms | 165/391 | 42.2 |
| Asthenia | 135/391 | 34.5 |
| Arthralgia | 94/391 | 24.0 |
| Weight loss | 73/391 | 18.7 |
| Fever | 53/391 | 13.6 |
| Myalgias | 31/391 | 7.9 |
| Löfgren syndrome | 89/391 | 22.8 |
| Asymptomatic | 40/396 | 10.1 |
| Thoracic involvement | 388/409 | 94.8 |
| Stage I | 138/409 | 33.7 |
| Stage II | 192/409 | 47.0 |
| Stage III | 34/409 | 8.4 |
| Stage IV | 23/409 | 5.7 |
| Extrathoracic involvement | 196/388 | 50.5 |
| Skin | 60/388 | 15.5 |
| Nonthoracic lymph node | 55/388 | 14.2 |
| Eyes | 53/388 | 13.7 |
| Liver | 26/388 | 6.7 |
| Spleen | 23/388 | 5.9 |
| Hypercalcemia/hypercalciuria | 15/388 | 3.9 |
| Neurological | 12/388 | 3.1 |
| Cardiac | 10/388 | 2.6 |
| Bone | 8/388 | 2.1 |
| Renal | 4/388 | 1.0 |
Thoracic involvement was detected in 94.8% of patients (Table 2). Based on the Scadding criteria, the majority of patients had stage I (33.7%) or stage II (47.0%) disease; 8.4% had stage III disease and 5.7% had stage IV disease.
Of the 388 patients with available lung function data, 54.4% had normal ventilation patterns, 16.9% had small airway obstruction, 13.6% had a restrictive pattern, 11.6% had an obstructive pattern, and 3.6% had a mixed pattern. Of the 45 patients with an obstructive pattern, 11 (24.4%) had history of smoking habits, however, after a judicious evaluation, the diagnosis of chronic obstructive pulmonary disease was not considered, and ventilatory changes were interpreted in the sarcoidosis context. Diffusion capacity for carbon monoxide (DLCO) was slightly reduced in 47.6% of the 286 patients with available data.
Extrathoracic involvementExtrathoracic involvement was observed in 50.5% of patients, 49.7% of whom were female and 52.2% of whom were male (P=0.58).
The most commonly affected organs were the skin (15.5%), the peripheral lymph nodes (14.2%), and the eyes (13.7%). From those with skin involvement, violaceous or erythematous maculopapular lesions were reported for 74.1% of patients and lupus pernio for 6.5%. Uveitis was the most common ocular manifestation of sarcoidosis (47.8% anterior and 20.5% posterior uveitis), followed by lacrimal gland involvement with onset of keratoconjunctivitis sicca syndrome (12.2%).
Potentially life threatening extrathoracic sarcoidosis, for example with cardiac and neurological involvement, were less common in this population, observed in 3.9% and 3.1% of the patients, respectively. Concerning neurological sarcoidosis (3.9%; n=12), 9 patients had mononeuropathy (6 with peripheral seventh nerve palsy – Bell's Palsy, 2 with optic nerve dysfunction, and 1 with peripheral mononeuropathy), 2 patients had hypothalamic involvement with diabetes insipidus and hypogonadism, and 1 patient developed acute aseptic meningitis. Regarding cardiac sarcoidosis (3.1%; n=8), 5 patients had frequent premature ventricular contractions and/or conduction system abnormalities and 3 patients had cardiomyopathy.
Frequencies of organ involvement are shown in Table 2.
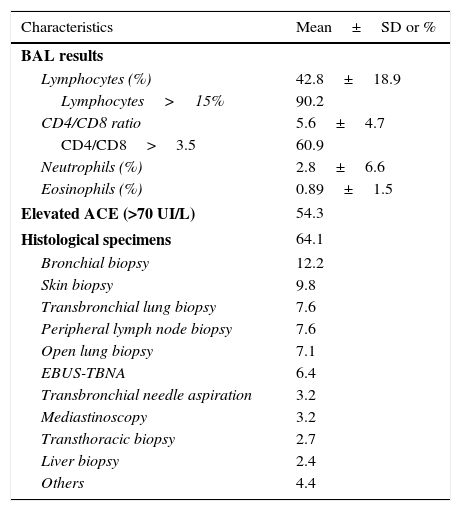
Diagnostic work-upBronchoscopic evaluation was performed in 289 patients; 65.7% had normal endoscopic findings, 25.3% had mucosal hyperemia, and 9.0% had granular mucosa. Differential cell counts in BAL fluid were available for 246 patients; of these, 90.2% had lymphocytosis (CD4/CD8 ratio ≥3.5 in 60.9% of cases).
Serum angiotensin-converting enzyme levels were assessed in 267 patients, and 54.3% had levels above the upper limit of normal (70IU/L).
In the diagnostic work-up, 64.1% of patients had a biopsy showing evidence of noncaseating epithelioid cell granulomas in histological specimens. The respiratory tract, the skin, and the peripheral lymph nodes were the most frequently biopsied organs. The diagnostic work-up results are summarized in Table 3.
Laboratory work-up results.
| Characteristics | Mean±SD or % |
|---|---|
| BAL results | |
| Lymphocytes (%) | 42.8±18.9 |
| Lymphocytes>15% | 90.2 |
| CD4/CD8 ratio | 5.6±4.7 |
| CD4/CD8>3.5 | 60.9 |
| Neutrophils (%) | 2.8±6.6 |
| Eosinophils (%) | 0.89±1.5 |
| Elevated ACE (>70 UI/L) | 54.3 |
| Histological specimens | 64.1 |
| Bronchial biopsy | 12.2 |
| Skin biopsy | 9.8 |
| Transbronchial lung biopsy | 7.6 |
| Peripheral lymph node biopsy | 7.6 |
| Open lung biopsy | 7.1 |
| EBUS-TBNA | 6.4 |
| Transbronchial needle aspiration | 3.2 |
| Mediastinoscopy | 3.2 |
| Transthoracic biopsy | 2.7 |
| Liver biopsy | 2.4 |
| Others | 4.4 |
BAL, bronchoalveolar lavage; ACE, angiotensin-converting enzyme; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
Systemic treatment was prescribed to 58.6% of patients. Complete resolution of disease was documented in 45.3% of patients during clinical and radiological follow-up; 97% of these had Scadding stages 0, I, or II and 60.0% experienced spontaneous resolution. The remaining 54.7% of patients developed chronic disease (63.4% showed few changes over time and 36.6% developed progressive disease). Four patients were referred for lung transplantation: one underwent single lung transplantation, one was rejected because of severe cardiac failure, and two died of pulmonary fibrosis with respiratory failure while waiting for transplantation.
DiscussionVery different clinical phenotypes have been reported for sarcoidosis in terms of presentation, organ involvement, and outcome.4,13,21 In our cohort of patients from northern Portugal, we found that sarcoidosis had a peak incidence in middle-aged individuals and was predominant in females (58.9% of cases). Our findings coincide with previous reports for other Caucasian populations,4,11,12,22,23 in which sarcoidosis was more common in middle-aged adults, with a peak between 30 and 39 years. We found no age-related differences between male and female patients. Women have been found to be older than men in some populations12,24–26 and in some regions, such as Scandinavia and Japan, a bimodal age distribution has been described, with peaks between 25–29 and 65–69 years of age.12,24,27 Sarcoidosis was more common in women in our cohort, with a female to male ratio of 1.4:1. This female preponderance has been reported across racial and ethnic groups.15,28–31
As expected, the prevalence of smokers and ex-smokers (24.5%) was lower than in the general Portuguese population (39.0%),32 supporting the hypothesis that smoking appears to exert a certain protective effect against the occurrence of sarcoidosis.33,34
Thoracic involvement was observed for 94.8% of patients in our cohort and about half had concomitant extrathoracic disease. These rates are similar to those described in the literature, including those described in the ACCESS study of 736 sarcoidosis patients from the United States.15 The presenting symptoms of sarcoidosis are diverse and their respective incidence depends on epidemiological factors.35 The most common manifestations in our cohort were respiratory symptoms, constitutional symptoms, and Löfgren syndrome. Although almost all the patients had lung involvement, only 59.2% mentioned respiratory symptoms (mainly, exertion dyspnea and dry cough). In other studies, respiratory symptoms were reported by 70% of patients in the United States15 and 73.7% of patients in Turkey.25 In the Netherlands, dyspnea was the main complaint in 70% of patients studied.31 Interestingly, two studies of sarcoidosis populations in Lisbon (Portugal) and Leon (Spain), which share genetic similarities with the population in northern Portugal, reported respiratory symptoms in just 7.3% and 20.3% of patients, respectively.23,30 Constitutional symptoms (mainly asthenia) were present in 42.2% of patients in our cohort. This is higher than the respective rates of 10.2% and 26.5% reported for Leon30 and Lisbon23 and lower than those reported for the Netherlands (71%)31 and Turkey (50.5%).25 These discrepancies could be related to differences in methodology or in type of care center, as a tertiary referral hospital would logically have a higher caseload of patients with more severe forms of disease than a primary care center or an epidemiological study of the general population. Löfgren syndrome was the presenting clinical form in 22.8% of patients and it was more common in females (44.4% vs 12.7% for males, P<0.001). Löfgren syndrome is linked to the HLA DRB1*03 allele,35,36 whose distribution varies considerably according to geographic location.38 This syndrome was reported in about 50% of patients on diagnosis of sarcoidosis in Spain,30,38,39 compared with just 2%–6% in Japan38,40 and 8% in the United States.15 The incidence in our series is similar to that reported for Scandinavian countries.14,24,25 Surprisingly high rates of Löfgren syndrome have been described for sarcoidosis patients in Spain and almost half of the cases were diagnosed during the spring months.30,38,39,41 Female predominance is a common observation for Löfgren syndrome.35,42 Sarcoidosis was diagnosed fortuitously on chest X-ray in 10.1% of patients (all asymptomatic), supporting previous reports suggesting that this form of diagnosis is not uncommon.35,43
A majority of patients had radiographic stages I and II disease (33.7% and 47.0%, respectively) according to the Scadding criteria. These rates are consistent with those reported for different populations.4,13,15,23,26,30,39,40 Lung function tests are usually part of routine investigation.4 The most common lung abnormality in our cohort was decreased DLCO, observed in 47.6% of patients. Lung volumes were normal in over half of the patients and when present, impaired respiratory function was mostly mild. Similar findings were reported in the ACCESS study15 and a previous Portuguese study.23 Lung function abnormalities tended obviously to be more marked from stages I to IV, as reported by other studies.44
Approximately half of the patients had extrathoracic involvement, mainly affecting the skin, the peripheral lymph nodes, and the eyes, as previously reported.2,15 The low prevalence of neurologic and cardiac involvement is also in line with data reported for Caucasian populations.4 Cardiac sarcoidosis is significantly more common in Japanese patients than in either white or African Americans.38,40Table 4 shows a summary of the features of sarcoidosis by geographic area according to a selection of studies.15,22–24,28,36–38,43,45
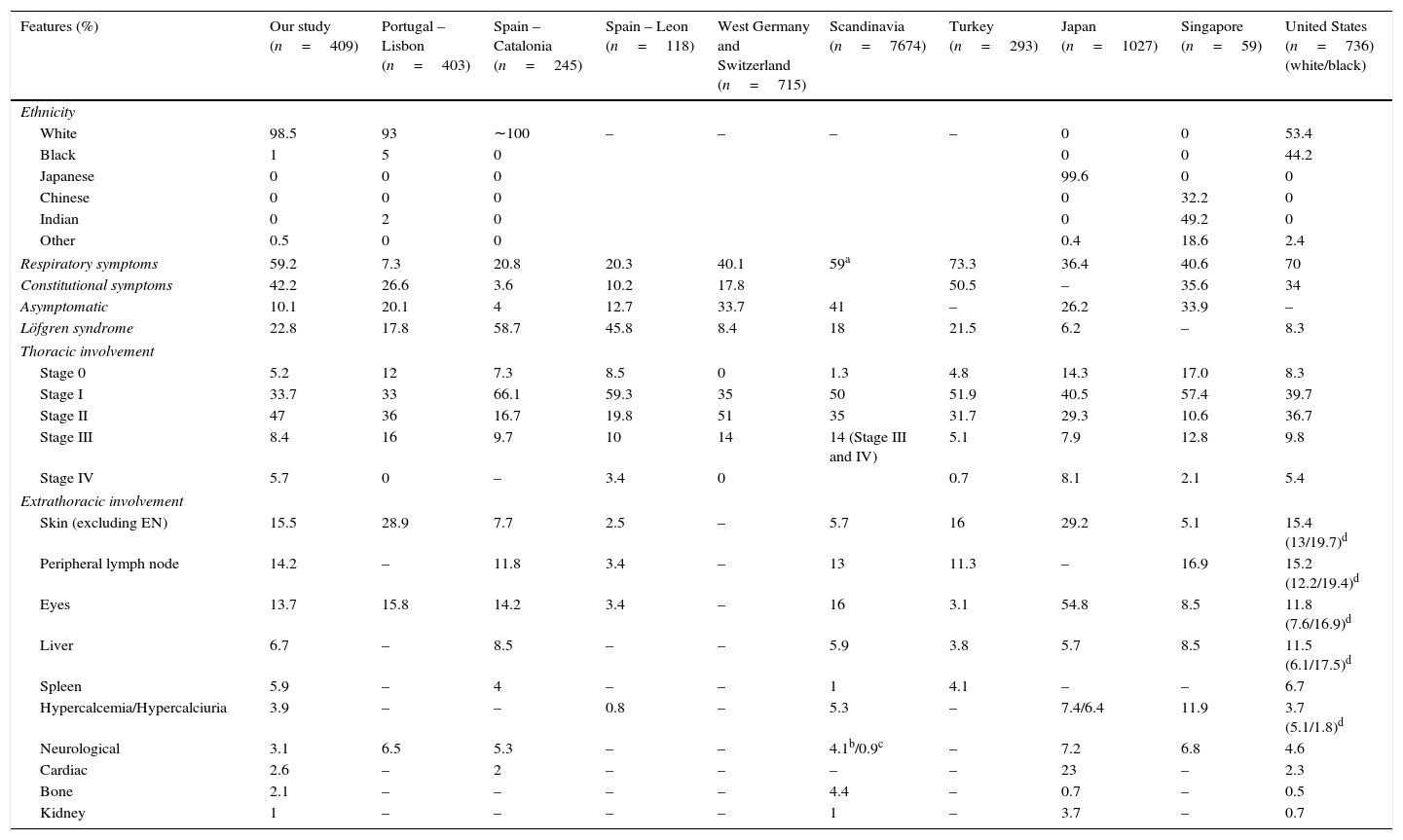
Features of sarcoidosis in selected geographic areas.15,22–24,28,36–38,43,45
| Features (%) | Our study (n=409) | Portugal – Lisbon (n=403) | Spain – Catalonia (n=245) | Spain – Leon (n=118) | West Germany and Switzerland (n=715) | Scandinavia (n=7674) | Turkey (n=293) | Japan (n=1027) | Singapore (n=59) | United States (n=736) (white/black) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethnicity | ||||||||||
| White | 98.5 | 93 | ∼100 | – | – | – | – | 0 | 0 | 53.4 |
| Black | 1 | 5 | 0 | 0 | 0 | 44.2 | ||||
| Japanese | 0 | 0 | 0 | 99.6 | 0 | 0 | ||||
| Chinese | 0 | 0 | 0 | 0 | 32.2 | 0 | ||||
| Indian | 0 | 2 | 0 | 0 | 49.2 | 0 | ||||
| Other | 0.5 | 0 | 0 | 0.4 | 18.6 | 2.4 | ||||
| Respiratory symptoms | 59.2 | 7.3 | 20.8 | 20.3 | 40.1 | 59a | 73.3 | 36.4 | 40.6 | 70 |
| Constitutional symptoms | 42.2 | 26.6 | 3.6 | 10.2 | 17.8 | 50.5 | – | 35.6 | 34 | |
| Asymptomatic | 10.1 | 20.1 | 4 | 12.7 | 33.7 | 41 | – | 26.2 | 33.9 | – |
| Löfgren syndrome | 22.8 | 17.8 | 58.7 | 45.8 | 8.4 | 18 | 21.5 | 6.2 | – | 8.3 |
| Thoracic involvement | ||||||||||
| Stage 0 | 5.2 | 12 | 7.3 | 8.5 | 0 | 1.3 | 4.8 | 14.3 | 17.0 | 8.3 |
| Stage I | 33.7 | 33 | 66.1 | 59.3 | 35 | 50 | 51.9 | 40.5 | 57.4 | 39.7 |
| Stage II | 47 | 36 | 16.7 | 19.8 | 51 | 35 | 31.7 | 29.3 | 10.6 | 36.7 |
| Stage III | 8.4 | 16 | 9.7 | 10 | 14 | 14 (Stage III and IV) | 5.1 | 7.9 | 12.8 | 9.8 |
| Stage IV | 5.7 | 0 | – | 3.4 | 0 | 0.7 | 8.1 | 2.1 | 5.4 | |
| Extrathoracic involvement | ||||||||||
| Skin (excluding EN) | 15.5 | 28.9 | 7.7 | 2.5 | – | 5.7 | 16 | 29.2 | 5.1 | 15.4 (13/19.7)d |
| Peripheral lymph node | 14.2 | – | 11.8 | 3.4 | – | 13 | 11.3 | – | 16.9 | 15.2 (12.2/19.4)d |
| Eyes | 13.7 | 15.8 | 14.2 | 3.4 | – | 16 | 3.1 | 54.8 | 8.5 | 11.8 (7.6/16.9)d |
| Liver | 6.7 | – | 8.5 | – | – | 5.9 | 3.8 | 5.7 | 8.5 | 11.5 (6.1/17.5)d |
| Spleen | 5.9 | – | 4 | – | – | 1 | 4.1 | – | – | 6.7 |
| Hypercalcemia/Hypercalciuria | 3.9 | – | – | 0.8 | – | 5.3 | – | 7.4/6.4 | 11.9 | 3.7 (5.1/1.8)d |
| Neurological | 3.1 | 6.5 | 5.3 | – | – | 4.1b/0.9c | – | 7.2 | 6.8 | 4.6 |
| Cardiac | 2.6 | – | 2 | – | – | – | – | 23 | – | 2.3 |
| Bone | 2.1 | – | – | – | – | 4.4 | – | 0.7 | – | 0.5 |
| Kidney | 1 | – | – | – | – | 1 | – | 3.7 | – | 0.7 |
EN, erythema nodosum.
Sarcoidosis remains a diagnosis of exclusion that is best supported by a tissue biopsy that demonstrates noncaseating granulomas in a patient with compatible clinical and radiologic features.2,4 However, some studies have suggested that lymphocytosis and a high CD4/CD8 ratio in BAL fluid may be sufficient to confirm diagnosis in patients with typical clinical/radiological features of sarcoidosis. A CD4/CD8 ratio ≥3.5 has a specificity of 93%–96% for sarcoidosis, although its sensitivity is considerably lower, at 53%–59%.46,47 In the present study, noncaseating granulomas were histologically confirmed in about two-thirds of patients and a CD4/CD8 ratio ≥3.5 in BAL fluid was observed in 60.9%.
Previous studies have reported spontaneous remission in nearly two-thirds of patients and chronic or progressive disease in 10%–30%.12,13,29,43 Approximately half of the patients in our cohort developed chronic forms of sarcoidosis, but only a third of these experienced disease progression. The higher proportion of chronic forms detected may be related to the fact that the cohort corresponded to patients from a tertiary care setting. This possible exclusion of less severe forms of sarcoidosis is a potential limitation of our study.
Several other limitations need to be mentioned. First, the retrospective design meant that not all data were available for all patients and also that we were missing certain data that could have shed light on the etiology of sarcoidosis (e.g., occupational and environmental exposures). Second, the study was not designed to capture all patients in our geographic area, and therefore we cannot comment on the overall incidence/prevalence of sarcoidosis in our region. Third, comparisons with findings from other studies also have potential pitfalls related to temporal and selection biases. Some of the variability between countries could be explained by differences in health beliefs or health-seeking behaviors (e.g., presence or not of chest X-ray screening programs for tuberculosis, primary, secondary or tertiary care centers context).
ConclusionWe have presented the first report of sarcoidosis cases in northern Portugal. In general, the epidemiological and clinical characteristics observed have similarities to those described for other Western European populations. However, we found a higher proportion of patients who progressed to chronic forms which is probably related with the fact that the study had been carried out in a tertiary university center. This study could become also a baseline for other epidemiological studies in Portugal about sarcoidosis. We believe that the population in the South of the country, with some genetic discrepancies and the presence of a black African community, could have different disease presentation, severity and evolution that would be interesting to make comparisons with. Indeed, large-scale multicenter studies are needed to gain more insight into the influence of ethnicity and geography on the incidence and clinical course of sarcoidosis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Funding sourceNo funding was obtained from any commercial or pharmaceutical companies. Funding for this study was supported by private donations.
Detail of authorship contributionsAll listed authors participated in the study conception, in the data acquisition/analysis and in the writing of the article.
Conflict of interest declarationThe authors have no conflicts of interest to disclose.