Asthma and Allergic Rhinitis (AR) are two chronic inflammatory diseases that are often concomitant. The Control of Allergic Rhinitis and Asthma Test (CARAT) was developed to evaluate the control of these diseases from the patients’ perspective. Its performance in asthma patients without AR has not been previously studied.
AimTo test the hypothesis that CARAT can be used to assess asthma control in patients with asthma and without AR.
MethodsA cross-sectional study was conducted in 3 primary healthcare centres in Northern Portugal. Adult patients identified in the Electronic Patient Record with a diagnosis of asthma were invited to participate. CARAT was used to assess asthma control and Asthma Control Test (ACT) as a comparator. The associations between asthma patients without AR (AsAR) and with AR (AwAR) were analyzed with Spearman correlation. Additionally, Receiver Operating Characteristic (ROC) curve analysis, summarized by Area Under the Curve (AUC), was used to assess performance of CARAT for screening asthma that was not well-controlled.
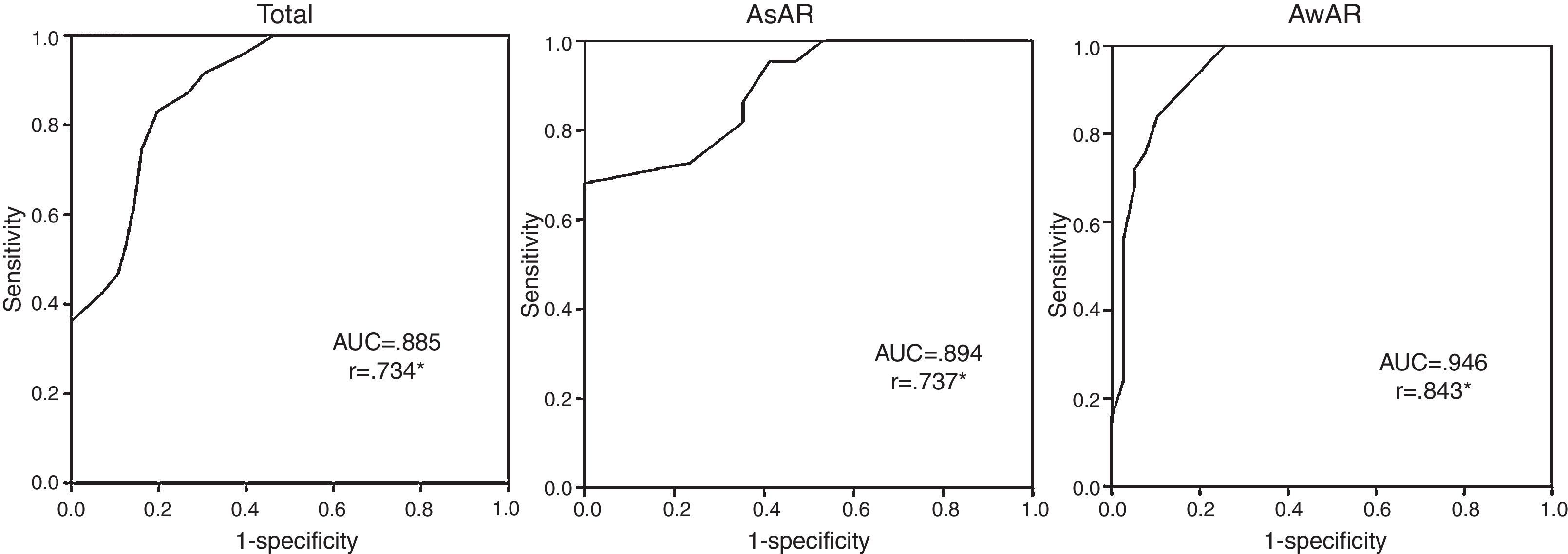
ResultsA total of 103 asthma patients completed the study, 64 (62%) had AwAR and in 87 (85%) asthma was not well-controlled. We observed a strong correlation between CARAT and ACT scores (r=0.734) in all asthma patients and in both groups: AsAR (r=0.737) and AwAR (r=0.843). ROC curve demonstrated CARAT as having a good discriminative power for both AsAR and AwAR groups (AUC=0.894 and 0.946, respectively).
ConclusionThese initial results suggest that CARAT has a good discriminative performance, similar to other asthma control assessment tools, for asthma patients with and without AR.
The “Allergic Rhinitis and its Impact on Asthma” (ARIA) highlights a close relationship between asthma and Allergic Rhinitis (AR) and recommends a combined approach to evaluate and manage these two conditions.1
The Control of Allergic Rhinitis and Asthma Test (CARAT) was the first questionnaire to assess the level of control of both asthma and AR using a single tool.2,3 CARAT was developed using a thorough formal methodological approach and the validation studies showed it has the good measurement properties observed fulfilling the ten items on the COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN).3 It is a Patient-Reported Outcome that quantifies the degree to which therapy goals are met in patients with a previous diagnosis of AR and asthma and can be used at all levels of care.4–6As CARAT's properties have been tested in patients with both asthma and rhinitis, the aim of this study was to test the hypothesis that CARAT can be used to assess asthma control in patients with asthma and without AR.
MethodsA cross-sectional study was conducted in Caldas das Taipas, a sub-urban area of Northern Portugal. All adult patients cared for in one of the three primary care centres (PCC), between November and December 2010, with an asthma diagnosis registered on the Electronic Patient Record (EPR), were eligible for the study. The exclusion criteria were: misdiagnosis/coding error confirmed by both the patient and the family doctor; physical or cognitive disabilities that prevented the completion of the questionnaire; patients that could not attend an appointment. A letter was sent to all eligible patients, explaining the aim of the project and inviting them to participate.
The Ethics Committee of the Regional Health Administration approved the study. Written informed consent was obtained from each participant.
Participants were classified has having AR based on the presence of two or more of five symptoms, as recommended by ARIA guidelines.1
CARAT has 10 questions and a rating scale from 0 to 30, with a cut-off value of 24 points to classify patients: not well-controlled (≤24) and controlled asthma (>24).4 The Asthma Control Test (ACT)7 was used as a comparator for asthma control using a cut-off of >19, well controlled, and ≤19, not well-controlled. For assessing AR symptoms, the Visual Analog Scale (VAS) was used, ranging from 0cm (good) to 10cm (bad), with a cut-off of 5.5cm for mild versus moderate/severe rhinitis.
Statistical analysis was performed using IBM SPSS v21 (Armonk, NY;IBM Corp.). The associations between CARAT with ACT scores in asthma patients without AR (AsAR) and with AR (AwAR) were analyzed by Spearman correlation. Receiver Operating Characteristic (ROC) curve analysis was used to assess the performance of CARAT score against ACT, summarized using the Area Under the Curve (AUC). A significance level of α<0.05 was considered. A power analysis for 2 independent proportions was conducted post hoc.
ResultsOf the 192 patients identified 103 participated – 29 could not be contacted, 5 refused to participate, 20 missed >1 appointments, 7 were misdiagnosed and 28 (15%) were unable to complete the questionnaire. The participation rate of eligible patients was 72%.
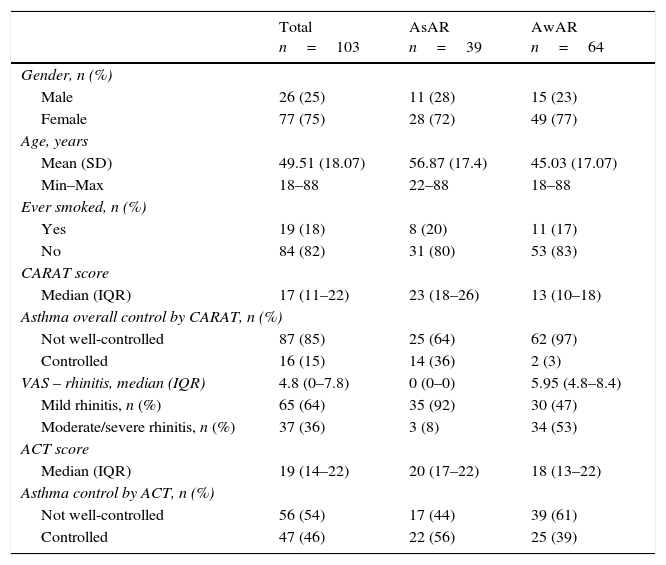
From the 103 patients included, 64 (62%) had AwAR (Table 1). Eighty-seven participants (85%) were classified as having not well-controlled asthma based on the CARAT score and 56 (54%) by the ACT score. In the group of AwAR a higher proportion were classified as having not well-controlled asthma both by CARAT and ACT, 97% and 61%, respectively, which compares with 64% and 44% in AsAR group (Table 1).
Characterization of all asthma patients and in both groups: AsAR and (AwAR).
| Total n=103 | AsAR n=39 | AwAR n=64 | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 26 (25) | 11 (28) | 15 (23) |
| Female | 77 (75) | 28 (72) | 49 (77) |
| Age, years | |||
| Mean (SD) | 49.51 (18.07) | 56.87 (17.4) | 45.03 (17.07) |
| Min–Max | 18–88 | 22–88 | 18–88 |
| Ever smoked, n (%) | |||
| Yes | 19 (18) | 8 (20) | 11 (17) |
| No | 84 (82) | 31 (80) | 53 (83) |
| CARAT score | |||
| Median (IQR) | 17 (11–22) | 23 (18–26) | 13 (10–18) |
| Asthma overall control by CARAT, n (%) | |||
| Not well-controlled | 87 (85) | 25 (64) | 62 (97) |
| Controlled | 16 (15) | 14 (36) | 2 (3) |
| VAS – rhinitis, median (IQR) | 4.8 (0–7.8) | 0 (0–0) | 5.95 (4.8–8.4) |
| Mild rhinitis, n (%) | 65 (64) | 35 (92) | 30 (47) |
| Moderate/severe rhinitis, n (%) | 37 (36) | 3 (8) | 34 (53) |
| ACT score | |||
| Median (IQR) | 19 (14–22) | 20 (17–22) | 18 (13–22) |
| Asthma control by ACT, n (%) | |||
| Not well-controlled | 56 (54) | 17 (44) | 39 (61) |
| Controlled | 47 (46) | 22 (56) | 25 (39) |
AsAR: asthma without allergic rhinitis; AwAR: asthma with allergic rhinitis; CARAT: Control of Allergic Rhinitis and Asthma Test; ACT: Asthma Control Test; VAS: Visual Analog Scale; SD: standard deviation; Min–Max: Minimum–Maximum; IQR: interquartile range.
There was a strong and significant correlation between ACT and CARAT scores in all asthma patients (r=0.734) and in both AsAR and AwAR groups (r=0.737 and r=0.843, respectively) (Fig. 1).
Receiver Operating Characteristic (ROC) curves and the respective correlation of CARAT score against ACT, for screening not well-controlled asthma, in all patients and both groups: AsAR and AwAR. AsAR: asthma without allergic rhinitis; AwAR: asthma with allergic rhinitis. AUC: Area Under the Curve; r de Spearman; * p<0.05.
The performance of CARAT for detecting not well-controlled asthma, using ACT as comparator was plotted as ROC curves and the respective AUC were 0.885 for all asthma patients, 0.894 for AsAR group and 0.946 for AwAR (Fig. 1).
DiscussionThis study was the first to test CARAT for evaluating asthma control in patients without AR. Overall our results suggest that CARAT performed well in assessing the level of asthma control regardless of whether patients had rhinitis or not. The correlations between CARAT and ACT were higher than 0.73 and the AUC higher than 0.88. These were slightly lower than those between Asthma Control Questionnaire (ACQ) and ACT (r=−0.87 to −0.89; AUCs=0.85–0.90) and similar to those between ACQ and Asthma Control Scoring System (ACSS).8–10 The difference observed in the proportion of control measured with CARAT and ACT was also reported in a study conducted in Portuguese pharmacies, suggesting that CARAT may have more sensitivity as an asthma control screening test in the community.6 A possible explanation for the differences in the classification of asthma control between CARAT and ACT may be the inclusion in ACT of the question “How would you rate your asthma control during the past 4 weeks?” In patients who do not perceive poor control, this question may decrease the sensitivity to detecting poor control. In fact, in the Portuguese Asthma survey we observed that 88% of not well-controlled asthma patients stated their disease was controlled when answering a question similar to the ACT question.11
The main limitation of this study is not having the physician's assessment of asthma control to compare with the CARAT score, however, this sample can potentially lead to generalizable results which can answer our study aim, as these patients were followed in primary care, by family doctors. ACT is a well-known asthma control assessment tool, used frequently in Portugal, even though no clinical validation study of the Portuguese (European) version has been published. The study has an adequate statistical power (1−β=0.662) and the participation rate was good for this type of study. However, the sample size is a limitation as it does not allow for further subgroup analysis. The inclusion of patients with a registered diagnosis of asthma in the EPR is both a strength and limitation of the study, because it reduces the probability of including patients with diseases other than asthma (specificity) and at the same time, many patients with asthma, perhaps less severe asthma, were probably not registered in the EPR. Finally, the high level of not well-controlled asthma observed may be partially explained by the study design. There is a need for studies conducted in different healthcare settings, in primary, secondary, tertiary care to assess the prevalence of uncontrolled asthma in different situations.
In conclusion, the results obtained from this study support the use of CARAT to evaluate the control of asthma not only in patients with rhinitis but also in patients without AR. Further studies are needed to confirm the applicability of CARAT in asthma patients without rhinitis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe study required no additional external funding. The statistical analysis was conducted with the support of the CARAT Project CINTESIS belonging to the Faculty of Medicine, University of Porto.
Conflicts of interestJoão A. Fonseca, MD, PhD is the coordinator of the CARAT project. The authors declare that they have no conflicts of interest in relation to this study.
The authors would like to acknowledge to Cláudia Camila Dias for help in the statistical analyses.