There are currently no reliable instruments for assessing the onset and progression of chronic obstructive pulmonary disease (COPD) or predicting its prognosis. Currently, a comprehensive assessment of COPD including several objective and subjective parameters is recommended. However, the lack of biomarkers precludes a correct assessment of COPD severity, which consequently hampers adequate therapeutic approaches and COPD control. In the absence of a definition of “well-controlled disease”, a consensus regarding COPD control will be difficult to reach. However, COPD patient assessment should be multidimensional, and anchored in five points: control of symptoms, decline of pulmonary function, levels of physical activity, exacerbations, and Quality of Life.
Several non-pharmacological and pharmacological measures are currently available to achieve disease control. Smoking cessation, vaccination, exercise training programs and pulmonary rehabilitation are recognized as important non-pharmacological measures but bronchodilators are the pivotal therapy in the control of COPD. This paper discusses several objective and subjective parameters that may bridge the gap between disease assessment and disease control. The authors conclude that, at present, it is not possible to reach a consensus regarding COPD control, essentially due to the lack of objective instruments to measure it. Some recommendations are set forth, but true COPD control awaits further objective assessments.
Traditionally, spirometry has been used to assess Chronic Obstructive Pulmonary Disease (COPD) severity and guide treatment, but a growing body of evidence documenting a poor correlation between forced expiratory volume in 1 second (FEV1) and symptoms or Quality of Life (QoL) in COPD patients,1–5 raises doubts as to the real effectiveness of spirometry as an instrument for assessing COPD control. Currently, several tools are recommended for a comprehensive evaluation of COPD, and although some are objective measurements, many remain subjective, such as questionnaires for symptoms or QoL assessment.5–12 This hampers COPD control: how can we define a well-controlled patient if a proper assessment, encompassing all its dimensions, is not currently attainable? Despite the existence of several pharmacological and non-pharmacological approaches to the management of COPD, there is a clear need to bridge the gap between disease assessment and disease control. This paper discusses several objective and subjective parameters that may make part of that bridge.
COPD: an evolving conceptThe first reference to COPD dates back to the XVII century, in the writings of Theophile Bonet, where he described the effects of lung emphysema as “voluminous lungs”.13 However, only almost three centuries later, in 1944, Ronald Christie wrote in the British Medical Journal that “[…] the diagnosis (of emphysema) should only be considered certain when dyspnoea on exertion, of insidious onset, not due to bronchospasm or left ventricular failure, appears in a patient who has some of the physical signs of emphysema together with chronic bronchitis or asthma.”.14 The CIBA Guest Symposium in 195915 and the American Thoracic Society Committee on Diagnostic Standards in 1962,16 were two landmark meetings that defined the components of COPD. In the 70s the risks of smoking, the accelerated rate of decline in FEV1 in susceptible smokers, and the effects of smoking cessation on lung function were elegantly described by Fletcher et al.17 During the 80s, focus was given to exacerbations, prophylactic antibiotic therapy and prevention. The standards for oxygen use in patients with advanced COPD and chronic respiratory failure were also defined in this decade. During the late 90s there was a marked evolution in COPD concepts, individual and social approaches, and guideline development. There was the emergence of old and new inhaled drugs, such as short- and long-acting β2-agonists (SABAs and LABAs), and the recognition of the role of corticosteroids, smoking cessation and pulmonary rehabilitation in the treatment of COPD, when there was still clinical confusion between asthma and COPD in terms of diagnosis and treatment.
It was not until the early XXI century that asthma and COPD were recognized as distinct pulmonary obstructive diseases, although the Asthma-COPD Overlap Syndrome (ACOS) has features of both pathologies. During the first decade of the XXI century, the role of each pharmacological class, namely long-acting muscarinic antagonists (LAMAs) and inhaled corticosteroids (ICSs), in COPD was established.18 LABAs were recognized as efficient in symptom relief, in improving quality of life and exercise tolerance, and in preventing exacerbations. Corticosteroids, on the other hand, have been considered to have a modest global effect on COPD, and their use remains controversial in stable patients, in particular due to evidence of increased risk of pneumonia.19 The central role of tobacco on COPD was strengthened, and widespread smoking cessation campaigns and programs were implemented. Techniques such as lung volume reduction (surgical or endoscopic) and transplantation were established for a small number of COPD patients. After 2000, the understanding of the social costs of COPD and the elevated disease-associated morbidity and mortality – higher than in asthma – led to population-based studies to determine its prevalence worldwide and its impact on mortality in developed countries. Although COPD prevalence varies across countries, ranging from 7.8% to 19.7%, and across different groups within countries, it is one of the most important causes of morbidity and mortality worldwide.10 Like hypertension or type 2 diabetes, COPD is a chronic disease, associated to modifiable risk factors, high morbidity and increased healthcare costs, and should thus be a target for disease control strategies. However, and contrary to hypertension or type 2 diabetes, these strategies are more difficult to implement in COPD given the difficulty in establishing objective criteria that may predict outcomes or decrease disease risk. Moreover, indicators of disease control and modifiers of disease progression are still to be defined.
After 2010, a considerable effort was made to establish different COPD phenotypes,20 and GOLD 2011 proposed different COPD stages.21 Recently, and in contrast with GOLD guidelines, it has been suggested that COPD management needs to be centered on disease stratification based on the risk of exacerbations and dyspnea symptoms.22 ICS finally found their niche in COPD, and are currently recommended for patients at high risk of exacerbation, whilst the addition of a second bronchodilator is recommended for symptomatic patients.10,22
COPD in now recognized as a systemic disease with pulmonary, cardiovascular, metabolic and musculoskeletal implications.
Initial assessment and symptom controlThere are currently no biomarkers to assess the onset of COPD, determine disease activity or severity or predict prognosis. Given the documented poor correlation between FEV1 and symptoms/QoL in COPD patients,1–5 several guidelines have recognized the need to incorporate non-spirometric measures in COPD assessment and in determining therapeutic options.6,7 Current GOLD guidelines recommend a comprehensive assessment of COPD, including symptoms, using validated questionnaires such as the modified Medical Research Council Dyspnea Scale (mMRC), the Clinical COPD Questionnaire (CCQ)8 and the COPD Assessment Test (CAT),9 degree of airflow limitation, risk of exacerbations and existence of co-morbidities.10 Other variables that should be taken into account in a comprehensive assessment of COPD are physical activity restrictions,5 particularly important because exercise capacity and functional status predict exacerbations, hospitalizations, and mortality.11 The overall impact of COPD in a patient's QoL should also be assessed. Some authors suggest that for the assessment of symptom control, clinical history, symptoms and spirometry should all be used.23
What is controlled COPD disease?One possible definition is “A patient with COPD is considered to be well controlled if, during follow-up, show minimal or no symptoms, no acute exacerbations have occurred since the last follow-up visit, and no impairment in QoL has been seen while receiving the current treatment”.12
However, in the absence of objective criteria, it is difficult to clearly define controlled disease. Is there an intermediate stage between controlled disease and uncontrolled disease? Not at present. Is a patient who has had one exacerbation equally controlled or uncontrolled compared with a patient who has experienced two or more exacerbations? At the moment, this question cannot be answered. Moreover, since COPD is a chronic progressive disease, one cannot expect a patient to remain completely asymptomatic. The physician's goal is to control the disease to the extent it can be controlled, and expectations of control will vary according to the individual and the COPD stage.
We propose that patients are considered to be well controlled if they are asymptomatic or show a decrease in symptoms from baseline, have stable or decreased decline of pulmonary function, show an increased tolerance to exercise, have no exacerbations and the best possible QoL.
Initial assessmentA clinical diagnosis of COPD should be considered in any patient who has dyspnea, chronic cough or sputum production, and a history of exposure to risk factors for the disease, with spirometry being required to establish a diagnosis.10 Although spirometric screening of asymptomatic individuals is not supported by evidence, in individuals over 40 years old and with a smoking history (>10 packs-year), spirometry may be performed with the aim of early diagnosis.24 However, as already stated above, there is a documented poor correlation between FEV1 and symptoms/QoL in COPD patients.1–5 Spirometry is not recommended during exacerbations because it can be difficult to perform and measurements are not accurate enough.10
Additional tests that can be useful in the differential diagnosis and characterization of COPD manifestations include chest X-ray, measurement of lung volumes and diffusing capacity for carbon monoxide (DLCO), and oxygen saturation levels at rest and during exercise.5 The use of Computed Tomography (CT) in the assessment of patients with COPD can provide quantitative measures of both emphysema and airway disease5 and it is useful in identifying the presence of previously unrecognized radiographic bronchiectasis that appears to be associated with longer exacerbations and increased mortality.10
We propose that initial disease severity assessment should include smoking history, symptoms (particularly dyspnea), spirometry, history of exacerbations, systemic manifestations, exercise tolerance, daily impact of the disease (health related QoL and health status) and mortality risk.
SpirometrySpirometry is required to establish a diagnosis of COPD: the presence of a post-bronchodilator FEV1/FVC<0.70 confirms the presence of persistent airflow limitation and is necessary for the diagnosis of COPD. FEV1 is one of the markers of disease severity, whereas FEV1 decline, among other biomarkers, should be evaluated to determine disease activity, related to disease progression and inflammation.25 In fact, the decline of pulmonary function across time may be a marker of uncontrolled disease and thus this should be taken into account. A normal value for spirometry effectively excludes the diagnosis of clinically relevant COPD.10 However, the documented poor correlation between FEV1 and symptoms/QoL in COPD patients,1–5 raises doubt about the real importance of spirometry as a tool for assessing COPD control. Spirometry should be used in symptomatic patients with risk factors for COPD.23
We agree that spirometry is required to establish a diagnosis of COPD.
We propose that spirometry is still performed once a year for monitoring COPD until new evidence or disease markers are available.
DyspneaDyspnea is a major cause of disability in COPD,10 it is the most prevalent symptom among patients with respiratory diseases,26 an independent predictor of mortality in patients with COPD,4,26 and associated with decreased exercise performance,27 physical performance, quality of life, anxiety and depression.3 It is the most restrictive symptom of COPD and reflects better overall disease impact than spirometry.3 Unfortunately, there are no objective measures of dyspnea. GOLD guidelines recommend the use of the above mentioned validated questionnaires.10 Again, all of these are subjective, but it is not possible to accurately stratify a subjective symptom. Despite their weaknesses, when used correctly, mMRC, CCQ and CAT are useful and feasible in clinical practice, and can be used in all consultations, both in primary care and hospital settings. However, mMRC may be preferable, given that it is simpler to use and is a better predictor of all-cause mortality in COPD patients compared to CCQ and CAT.28
Although there is no doubt that dyspnea is the cardinal symptom of COPD, others such as cough and sputum production should not be underestimated. Cough is often neglected by healthcare professionals and ignored in population studies, but, together with sputum production, has a negative impact on the patient's QoL. A large study in patients with severe COPD showed that 58.7% of patients experience mild to severe cough and 63.6% mild to severe sputum production.29 Moreover, chronic mucus hypersecretion is significantly associated with FEV1 decline and risk of subsequent hospitalization because of COPD,30 and increases the risk of pneumonia31 therefore, should not be overlooked but be part of symptom assessment.
We recommend that COPD symptom assessment should focus mainly on dyspnea, although cough and sputum production should not be underestimated. Assessment of dyspnea may be achieved by using mMRC, CCQ and CAT at every consultation, but if this is not possible, preference should be given to mMRC.
Physical activityInactivity is associated with a greater lung function decline, which can be partially reversed by exercise,32 and low levels of physical activity predict all-cause mortality in patients with COPD.33 Current guidelines recommend that all patients with COPD should engage in regular physical activity regardless of disease severity,6,7,10,20,24 also recommending that any training program should be tailored to each individual patient.
We agree with current guidelines on physical activity recommendations for COPD patients.
ExacerbationsExacerbations contribute to the overall severity of COPD,10,24 to the pulmonary function decline and the impairment of QoL,12,34–37 to morbidity38 and mortality.34–38 Exacerbations also increase the risk of cardiovascular disease, of developing further exacerbations, contribute to reduce muscle mass,35 limit physical activity,35,36 increase anxiety, depression, work absenteeism, and healthcare costs.35,37 Exacerbations may occur regardless of the degree of functional impairment,12,36,39,40 and it has been shown that mild and moderate exacerbations, often unreported and thus untreated, also affect health status.12,36 Therefore, it is important to predict and promptly identify exacerbations, and assess their severity and number,12,34 in order to appropriately manage them, prevent hospitalization,34,35 increase the patient's QoL and reduce the high costs associated with their treatment.24
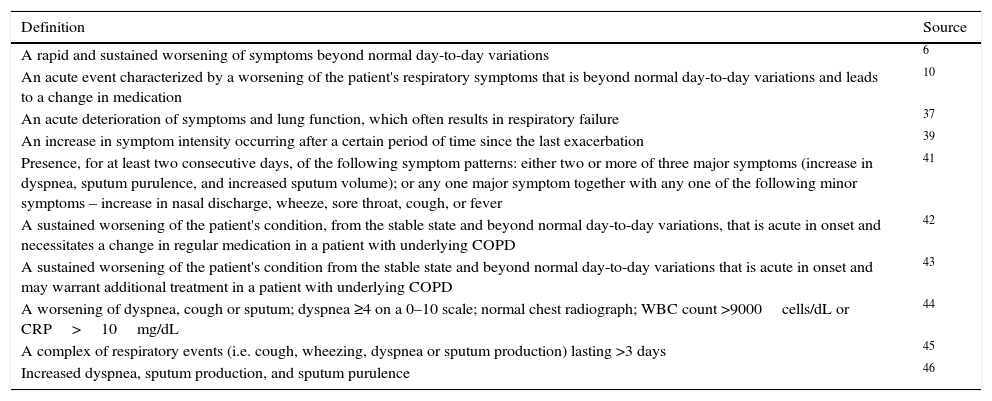
The first step to achieving these goals would be through a consensus definition of exacerbation. However, in the absence of easily quantifiable criteria,35 there is no exact or consistent definition of exacerbation,38 and several definitions have been implemented6,10,37,39,41–46Table 1. Also, a consensual and universal classification system to assess the severity of an exacerbation is lacking, although some have been proposed.10,42,43
Some currently used definitions of an exacerbation of COPD.
| Definition | Source |
|---|---|
| A rapid and sustained worsening of symptoms beyond normal day-to-day variations | 6 |
| An acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication | 10 |
| An acute deterioration of symptoms and lung function, which often results in respiratory failure | 37 |
| An increase in symptom intensity occurring after a certain period of time since the last exacerbation | 39 |
| Presence, for at least two consecutive days, of the following symptom patterns: either two or more of three major symptoms (increase in dyspnea, sputum purulence, and increased sputum volume); or any one major symptom together with any one of the following minor symptoms – increase in nasal discharge, wheeze, sore throat, cough, or fever | 41 |
| A sustained worsening of the patient's condition, from the stable state and beyond normal day-to-day variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD | 42 |
| A sustained worsening of the patient's condition from the stable state and beyond normal day-to-day variations that is acute in onset and may warrant additional treatment in a patient with underlying COPD | 43 |
| A worsening of dyspnea, cough or sputum; dyspnea ≥4 on a 0–10 scale; normal chest radiograph; WBC count >9000cells/dL or CRP>10mg/dL | 44 |
| A complex of respiratory events (i.e. cough, wheezing, dyspnea or sputum production) lasting >3 days | 45 |
| Increased dyspnea, sputum production, and sputum purulence | 46 |
COPD – Chronic Obstructive Pulmonary Disease; WBC – White Blood Cell; CRP – C-reactive protein.
The question of how to predict or identify a potential exacerbation during a routine follow-up visit remains unanswered. What are the known risk factors? Are there objective biological and clinical markers? Are questionnaires helpful?
Numerous risk factors for the occurrence of exacerbations are identified.10,12,36,37 GOLD recommends several objective tests to assess the severity of an exacerbation,10 and other authors suggest additional assessments.12,36 Questionnaires such as the mMRC, CCQ and CAT11 may also be helpful in this evaluation.
We propose that an exacerbation should be defined as a sustained acute/subacute worsening of the severity or frequency of symptoms such as dyspnea, cough or sputum production, with increased QoL impairment, lasting at least 3 days, which prompts the patient to seek medical attention or leads to a change in medication.
Quality of LifeAll the recommended QoL questionnaires have weak points.11,12 They are not thorough, and there may be others symptoms and factors that influence QoL. Also, patients tend to dislike completing questionnaires, preferring instead to talk to their doctor, and clinicians may also find it difficult to complete questionnaires in daily clinical practice.11 Currently, there are no alternative means for assessing symptoms and QoL, but it would be desirable to have a more comprehensive, yet easy and quick to use, assessment of all the factors that impair these patients’ global health.
Non-pharmacological measures to achieve COPD controlRecommended non-pharmacologic management of COPD depends on the individualized assessment of symptoms and exacerbation risk. GOLD proposes as essential smoking cessation, physical activity, influenza and pneumococcal vaccination for all patient groups, and pulmonary rehabilitation for B, C and D patients.10 Additional non-pharmacological measures include patient education, an appropriate diet,12,47 oxygen therapy and ventilatory support.47
Smoking cessationSmoking cessation has the greatest capacity to influence the natural history of COPD,24,48 it is the most effective way of preventing or delaying the development of airflow limitation and reducing disease progression,47 it improves symptoms and, at the moment, it is the best intervention to decrease COPD associated mortality.49 Therefore, it is imperative to have a proper and adequate support structure to encourage and help patients to quit smoking. However, COPD smokers require special support in order to quit smoking, and it has been reported that 33% to 50% of COPD patients remain smokers18,50 despite being strongly advised to quit smoking due to their COPD. Cigarette smoking should be regarded as a chronic relapsing disease, and the role of the clinician is to help smokers achieve abstinence (remission), by recognizing that relapses may occur. Tobacco dependence treatments are cost-effective relative to other medical and disease prevention interventions.51 Although brief tobacco dependence treatment has been found to be effective, there is a strong dose–response relationship between the intensity of tobacco dependence counseling and its effectiveness.52
Nicotine replacement therapy, as well as pharmacotherapy with varenicline or bupropion, reliably increases long-term smoking abstinence rates.10 An intensive and prolonged relapse prevention program is also recommended.24,47
VaccinationIn the age groups ≥50 and ≥65, hospitalization for Community Acquired Pneumonia (CAP) represents 5.5% and 7.0%, respectively, of total admissions for all causes in Portugal.53 Therefore, influenza and pneumococcal vaccination are recommended.6,7,10,20,24,53,54 In Portugal two pneumococcal vaccines are available, a pneumococcal polysaccharide vaccine 23-valente and a pneumococcal conjugate vaccine 13-valente. COPD patients must follow the dosing schedule suggested.53
Physical activityPatients with COPD have significantly lower levels of physical activity compared to healthy controls,55 and breathlessness on exertion, the primary symptom limiting exercise, leads to a reduced physical activity in these patients.56 Physical activity is dramatically reduced during and after hospitalization due to a COPD exacerbation, and this initiates a vicious cycle, since increased duration of inactivity promotes new exacerbations and hospitalizations.57 Moreover, evidence suggests that reduced physical activity predisposes to greater incidence of cardiovascular diseases, type 2 diabetes, cancer, dementia, physical disability and depression, conditions that are common co-morbidities of COPD.58 Exercise training programs improve exercise capacity, dyspnea and fatigue. Lower-limb training (e.g., walking or static bicycle) is optimally effective and provides the greatest short-term benefit. It improves exercise tolerance, reduces the number of exacerbations and hospitalizations, and improves QoL. The amount of regular maintenance physical activity recommended is walking 30–45min/day three-times a week followed by climbing up and down the stairs several times for 5minutes.12 However, the amount of regular physical activity needed to obtain a significant effect on admissions due to COPD is equivalent to walking or cycling for 2h per week.59
COPD patients are often limited in their ability to perform exercise and several adjunct therapies have been proposed for use during exercise, namely Non-Invasive Ventilatory Support (NIVS) and Heliox.27 Also, increase in exercise capacity with rehabilitation programs in combination with behavioral change may have the potential to increase physical activity in patients with COPD.60 Indeed, increasing the exercise capacity of patients with COPD may be insufficient to increase participation in leisure time activity. Interventions including self-monitoring of activity behavior using activity monitors in combination with behavioral counseling in patients with COPD might have the potential to change physical activity behavior. Key components that increase the effectiveness of behavioral interventions have already been summarized in several meta-analyses, international guidelines and reviews.61
Pulmonary rehabilitationPulmonary rehabilitation (PR) is a comprehensive intervention based on a complete patient assessment followed by individual therapies, which include, but are not limited to, exercise training, education, and behavior change. It is designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors.62
Exercise training is considered to be a crucial element of the rehabilitation program. It enhances exercise tolerance mainly through improvement of skeletal muscle function and reduction of ventilatory requirements during exercise, and increases functional performance through physiologic improvements, enhanced movement efficiency, and perhaps increased self-efficacy.63 The length of the exercise training component ranges from 4 to 10 weeks and the longer the program continues, the more effective the results.10 Some authors suggest a minimum duration of 8 weeks with a minimum frequency of three training sessions a week.64 Lower-limb training is the most important goal to achieve during pulmonary rehabilitation of these patients.12,47
However, it is of the utmost importance to maintain the benefits of an exercise training program in the long term, which will perhaps have an impact on truly modifying the long-term non-respiratory consequences of COPD.65
Rehabilitation has several benefits: (a) improves patient-reported outcomes, such as symptoms and QoL63; (b) leads to psychological improvements66; and (c) decreases the use of health care resources.64 PR benefits appear to decline 12 months after the end of the intervention.67
Further research should focus on strategies such as behavioral changes, to ensure the long-term benefits of rehabilitation programs for patients with COPD.
It is consensual that non-pharmacological measures have a pivotal role in disease control. They are useful, necessary and effective.
However, we acknowledge that these measures are difficult to implement at an individual level, not only due to their impact on lifestyle changes but also because they are often underestimated by patients. The lack of nationwide information campaigns and concerted strategies also hamper the implementation of these measures.
We propose that nationwide campaigns and strategies promoting the necessary conditions to design an optimized personalized plan for each patient, considering disease stratification, patient teaching, coordination with rehabilitation facilities, rehabilitation medicine, gymnasiums, and other possible support structures, should be implemented.
Pharmacological measures to achieve COPD controlRecommendations for therapy are set forth by international guidelines.6,7,10,24 There are three main therapeutic goals in COPD: (1) reduce symptoms; (2) reduce risk, and (3) improve prognosis.10 Beyond the already discussed non-pharmacological measures, several pharmacological approaches are currently available to manage stable COPD. The choice of pharmacotherapy should be based on symptoms, exacerbations and severity of obstruction. Inadequately controlled symptoms or the presence of exacerbations may modify therapeutic stratification.
Bronchodilators are the pivotal therapy in the control of COPD. According to the GOLD guidelines, inhaled formulations are preferable to systemic formulations due to less adverse events and increased efficacy. Also, long acting bronchodilators are preferable to short acting ones. The choice between a LABA or a LAMA depends on availability and individual response to therapy. The association of bronchodilators of different classes, e.g. LABA+LAMA, may improve efficacy and safety compared to dose adjustment of a bronchodilator in monotherapy. Indeed, a recently approved fixed-dose LABA/LAMA combination was associated with the concept of disease control.68–71 ICS should only be used in patients at higher risk of exacerbations and never as monotherapy, only added after trials of one or more long-acting bronchodilators, and only in frequent exacerbators (≥2 exacerbations per year or 1 exacerbation with hospitalization).10 In addition, and according to some data, a FEV1<60% should also be taken into consideration.18
However, the recognized heterogeneity of COPD has therapeutic implications. The distribution of variables such as severity of airflow limitation, degree of breathlessness, health status, presence of co-morbidities, exercise capacity, and number of exacerbations in the previous year has been reported to be highly variable within each GOLD stage.1 Also, classification of disease severity by airflow obstruction has been challenged not only by GOLD guidelines10 but also by other studies.72,73 This variability precludes a general therapeutic approach for COPD patients. Indeed, the Spanish guidelines for treatment of COPD proposes four clinical phenotypes on which pharmacological treatment should be based, and recommends different treatment options depending on these four phenotypes.74,75 These guidelines have been recently updated, with some modifications.20
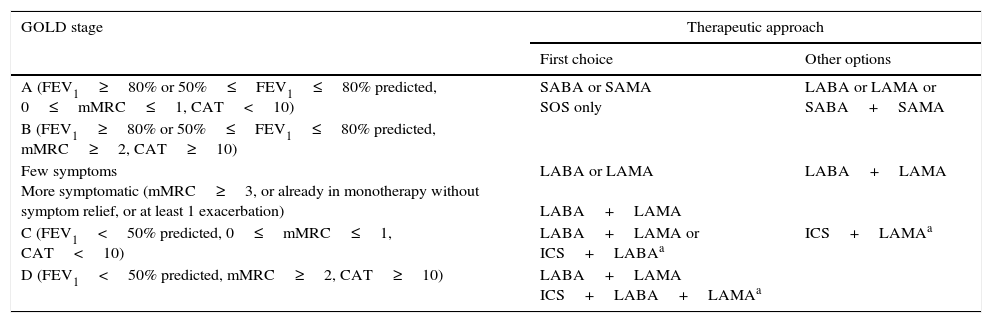
We suggest a pharmacological therapeutic approach based on GOLD stages Table 2.
Proposed pharmacological therapeutic approach according to GOLD stage.
| GOLD stage | Therapeutic approach | |
|---|---|---|
| First choice | Other options | |
| A (FEV1≥80% or 50%≤FEV1≤80% predicted, 0≤mMRC≤1, CAT<10) | SABA or SAMA SOS only | LABA or LAMA or SABA+SAMA |
| B (FEV1≥80% or 50%≤FEV1≤80% predicted, mMRC≥2, CAT≥10) | ||
| Few symptoms More symptomatic (mMRC≥3, or already in monotherapy without symptom relief, or at least 1 exacerbation) | LABA or LAMA LABA+LAMA | LABA+LAMA |
| C (FEV1<50% predicted, 0≤mMRC≤1, CAT<10) | LABA+LAMA or ICS+LABAa | ICS+LAMAa |
| D (FEV1<50% predicted, mMRC≥2, CAT≥10) | LABA+LAMA ICS+LABA+LAMAa | |
Note: Other pharmacological therapies, namely xanthines, aminophylline and theophylline, are available and may be useful in some patients. GOLD – Global initiative for chronic Obstructive Lung Disease; mMRC – modified Medical Research Council dyspnea scale; CAT – COPD Assessment Test; SABA – short-acting beta agonist; SAMA – short-acting muscarinic antagonist; LABA – long acting β2-agonist; LAMA – long-acting muscarinic antagonist; ICS – inhaled corticosteroid.
Pharmacological treatment should not be given to asymptomatic GOLD A patients, but a SABA or a short-acting muscarinic antagonist (SAMA) can be used as rescue medication, if needed. However, if patients have a low FEV1 (50%≤FEV1≤80% predicted), evidence of hyperinflation, and mMRC=1, a long acting bronchodilator for symptom relief is recommended.10 For GOLD B patients, the choice of LABA or LAMA should be made according to efficacy in each individual patient. However, since previous exacerbations are the most reliable predictors of future exacerbations, the choice of a single long-acting bronchodilator should take into account the decreased risk of exacerbations,76 and, in this context, a LAMA should be the first choice. The combination LABA+LAMA is recommended for more symptomatic patients. Maintenance of monotherapy before switching to dual bronchodilation should be based on symptoms, risk factors, and possibility of improving non-pharmacological measures. There is no evidence concerning ICS treatment in patients with FEV1>60% predicted.10 For GOLD C and D patients, the combination LABA+LAMA should be the first choice; for those patients with frequent exacerbations (≥2 exacerbations/year, or 1 exacerbation with hospitalization), the ICS/LABA combination should be preferred. Triple therapy with ICS+LAMA+LABA should be considered for patients with frequent exacerbations who continue to have symptoms. Although there have been some developments on the inclusion of eosinophilic counts in the decision tree to prescribe ICS, this paper does not discuss this issue, which is duly addressed elsewhere.77
There are some additional therapies not mentioned in Table 2 that are available and may be useful in certain patients. In patients with severe hereditary α-1 antitrypsin deficiency (ZZ genotype), α-1 antitrypsin replacement therapy may be considered for young patients with established emphysema who meet the established laboratory criteria.10 However, this therapy is very expensive and is not available in most countries.10 The National Institute for Health and Care Excellence (NICE) guidelines do not recommend replacement therapy in patients with α-1 antitrypsin deficiency.6 Another class of potentially useful drugs is mucolytic agents, such as N-acetylcysteine (NAC) or carbocysteine, but their effect on reducing exacerbations have not been consistent across studies,78–85 and seem to depend on dose and COPD severity. Therefore, their generalized use is not recommended. Erdosteine seems to be associated with a significant benefit in terms of symptom amelioration,86 but larger long-term studies with fully validated endpoints are required87 before a clear recommendation can be offered. Theophylline, a methylxanthine, should only be considered if other long-term treatment bronchodilators are unavailable or unaffordable, or in patients who are unable to use inhaled therapy. Intravenous methylxanthines (theophylline or aminophylline) should only be used in the management of exacerbations when there is insufficient response to short-acting bronchodilators.6,10,24 The available evidence on the efficacy of immunostimulating agents is currently not supportive of a recommendation.10
We agree that bronchodilation is the cornerstone of COPD treatment. COPD is a highly variable disease, and each individual patient will have different therapeutic needs. Therefore, it is imperative to tailor therapy to each patient, given an optimized therapeutic approach will not only improve symptoms but also increase compliance. We further propose that exacerbator phenotypes modulate COPD severity within each GOLD stage and should therefore have a more aggressive treatment approach.
MonitoringSeveral key points have been proposed to be included on the follow-up of COPD patients.12 One important aspect is that COPD patients often underestimate their symptoms, namely dyspnea, since they adapt their lifestyle to cope with it, which may lead to undertreatment. Therefore, measuring symptoms in a routine manner using questionnaires, can lead to a better understanding of the patient's overall clinical status and hence to the adjustment of treatment accordingly. In recent years, the goal of COPD management has shifted toward optimizing symptom control and reducing future risk, such as exacerbations, mortality, and co-morbidities, as well as the long-term consequences of COPD. While prevention and treatment of symptoms may not preclude long-term lung function decline, symptom control could provide measurable improvements in other key outcomes.11
There are no clear recommendations concerning when COPD patients should be referred to a pulmonology outpatient department, but some guidelines suggest reasons for referral, e.g. FEV1<50% predicted, frequent exacerbators, α-1 antitrypsin deficiency, ACOS, or uncertain diagnosis.6,7,24
In the absence of clear recommendations, we propose that a key aspect of COPD monitoring resides in a multidisciplinary approach, with Primary Care having a central role in the monitoring and follow-up of the majority of stable COPD patients. Primary Care should be responsible for recognizing when a patient should be referred to a specific specialty, e.g. Pulmonology, Cardiology, Internal Medicine or Physical Rehabilitation, and henceforth the coordinated and integrated management of comorbities should be achieved.
There is no established time interval between follow-up consultations, and this should be guided by clinical judgment. We recommend that a stable COPD patient, with no exacerbations and who complies with therapy, may have a follow-up consultation every 6 months. In all follow-up consultations questionnaires should be completed, especially mMRC. Annual spirometric assessment is recommended, especially in poorly controlled patients and frequent exacerbators. Although oximetry is useful, it is conditioned by availability.
ConclusionsPresently, it is not possible to reach a consensus regarding COPD control, which will probably be difficult to attain until a consensus definition of “well-controlled disease” is achieved. However, this definition depends not only on a thorough assessment of COPD severity and symptoms, but also their individual and social impact, which, again, are lacking. Initial assessment, monitoring and follow-up of COPD patients do not yet have clear recommendations, and new, more accurate, instruments to assess disease control are missing. Consensus about COPD control will probably not be attainable before the gap between disease assessment and disease control is bridged. Once this is achieved, it may pave the way for new avenues of research that will eventually answer the current questions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Role of funding sourceFunding for this paper was provided by Novartis Portugal. Funding was used to access all necessary scientific bibliography and cover meeting expenses. Novartis Portugal had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.
Conflict of interestThe authors declare collaborating and receiving fees from pharmaceutical companies other than Novartis either through participation in advisory board or consultancy meetings, congress symposia, clinical trial conduct or investigator-initiated trials.
The authors wish to thank Novartis Portugal for the funding of this paper, which was used to access all necessary scientific bibliography and cover meeting expenses.