To determine whether the duration of respiratory distress symptoms in severe COVID-19 pneumonia affects the need for invasive mechanical ventilation and clinical outcomes.
Materials and methodsAn observational multicentre cohort study of patients hospitalised in five COVID-19–designated ICUs of the University Hospitals of Emilia-Romagna Region. Patients included were adults with pneumonia due to SARS-CoV-2 with PaO₂/FiO₂ ratio <300 mmHg, respiratory distress symptoms, and need for mechanical ventilation (invasive or non-invasive). Exclusion criteria were an uncertain time of respiratory distress, end-of-life decision, and mechanical respiratory support before hospital admission.
Measurements and main resultsWe analysed 171 patients stratified into tertiles according to respiratory distress duration (distress time, DT) before application of mechanical ventilation support. The rate of patients requiring invasive mechanical ventilation was significantly different (p < 0.001) among the tertiles: 17/57 patients in the shortest duration, 29/57 in the intermediate duration, and 40/57 in the longest duration. The respiratory distress time significantly increased the risk of invasive ventilation in the univariate analysis (OR 5.5 [CI 2.48–12.35], p = 0.003). Multivariable regression analysis confirmed this association (OR 10.7 [CI 2.89–39.41], p < 0.001). Clinical outcomes (mortality and hospital stay) did not show significant differences between DT tertiles.
DiscussionAlbeit preliminary and retrospective, our data raised the hypothesis that the duration of respiratory distress symptoms may play a role in COVID-19 patients’ need for invasive mechanical ventilation. Furthermore, our observations suggested that specific strategies may be directed towards identifying and managing early symptoms of respiratory distress, regardless of the levels of hypoxemia and the severity of the dyspnoea itself.
‘Silent or happy hypoxia’, i.e., severe hypoxemia without signs of respiratory distress,1 has been repeatedly observed in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia,2 while the association between respiratory distress and the need for invasive mechanical ventilation (MV) has been poorly investigated. To the best of our knowledge, the duration of respiratory distress and the respiratory support progress in coronavirus disease 2019 (COVID-19) hypoxic patients has not been addressed. Hence, we aimed to investigate the association between the time elapsed from the onset of distress to the onset of ventilatory support in COVID-19 patients.
Materials and methodsWe analysed all consecutive patients with a diagnosis of interstitial SARS-CoV-2 pneumonia admitted to the tertiary university hospitals of the Emilia-Romagna region (Modena, Parma, and Bologna) over a nine-month period. The Ethical Committees of each hospital (658/2020/OSS*/AOUMO SIRER ID 417) approved the study. Inclusion criteria were age older than 18 years; PaO₂/FiO₂ ratio (defined as the ratio of the partial pressure of arterial oxygen to the percentage of inspired oxygen) <300 mmHg; and development of in-hospital respiratory distress, which was based on a respiratory rate >30 breaths per minute, subjective breathing with severe discomfort and accessory respiratory muscle use, and the need for invasive or non-invasive MV during the hospital stay. Exclusion criteria were an uncertain time of respiratory distress; onset of respiratory distress during the pre-hospital period; end-of-life decision; and mechanical respiratory support prior to hospital admission. Distress time (DT) was defined as the time elapsed from the onset of respiratory distress to the onset of positive pressure ventilation (MV either invasive or non-invasive), and the end of DT was declared when the patient reported that they no longer had severe respiratory discomfort and when they clinically stopped using the accessory breathing muscles.
All patients admitted to the five ICUs of the participating centres received standard of care treatment according to the Regional COVID-19 Guidelines3 during the study period, with minor changes in terms of compassionate use of Remdesivir and cytokine blocking agents. In management protocols, the indications for non-invasive MV included a PaO2/FiO2 ratio less than 200 mmHg, respiratory rate of 30 or more breaths per minute, and respiratory distress with activation of accessory respiratory muscles, while indications for invasive MV were neurological failure (altered consciousness with a Glasgow Coma Scale score of <10), cardiovascular failure (vasopressor requirement or major electrocardiogram changes, including arrhythmia or changes in repolarisation phase), and persistence of severe respiratory distress and hypoxemia with the need for FiO2 at 80% or higher to maintain a SaO2 level of 90%, or a PaO2/FiO2 ratio above 100 mmHg after a non-invasive MV trial. The COVID-19 team assessment included the multidisciplinary patient's assessment by an infectious disease specialist, pulmonologist, and intensivist.
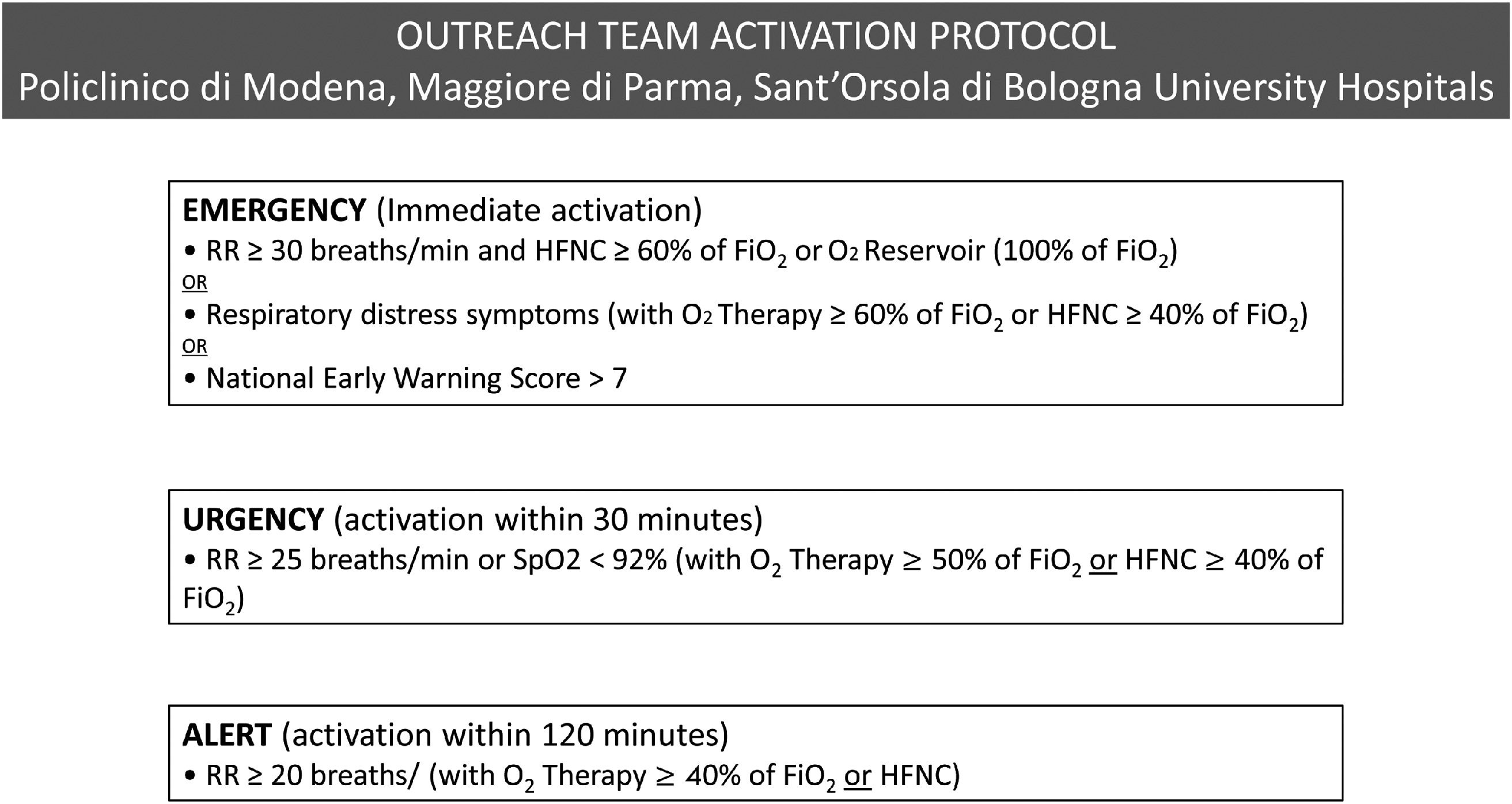
Demographic characteristics, comorbidities, laboratory and respiratory parameters and DT were collected by intensivists, unaware of the analysis, from the electronic chart of each patient. The beginning of the DT was certified in the patient's electronic chart by the outreach team (intensivists and pulmonologists), activated as “emergency call” from the infectious disease colleagues based on a specific protocol shared between the hospitals (Fig. 1). The relationship between DT and the need for invasive MV was evaluated by stratifying patients into tertiles according to the DT distribution. Likewise, 30-day mortality, hospital mortality, and hospital-free days at day 90 were also evaluated based on DT distribution. The association between the need of invasive MV and DT was estimated by unadjusted analysis and a multivariable regression analysis, including variables with p < 0.2 at unadjusted analysis in the model.
Continuous variables were expressed as median and interquartile ranges while categorical variables were reported as numbers and percentages. A comparison between variables was performed through the Kruskal Wallis test for continuous variables and the Chi-square test for categorical variables. Statistical significance was set as p < 0.05.
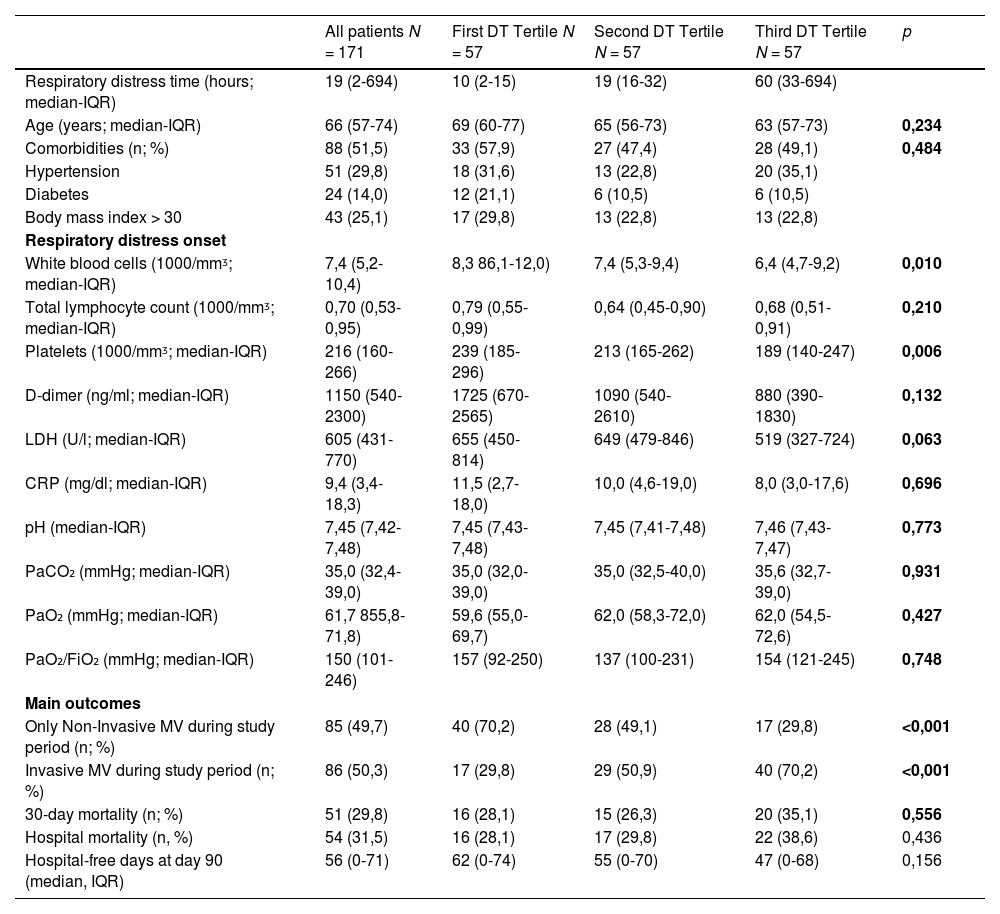
ResultsDuring the study period, 1415 patients were hospitalized for COVID-19, of whom 309 needed MV support during the hospital stay; among MV patients, 171 met inclusion criteria and were included in the study. White blood cells were lower (p = 0.010) at DT onset in the third tertile, as was platelet count (p = 0.006) (Table 1).
Patients’ characteristics and parameters at respiratory distress onset and the initiation of the ventilatory support stratified for the respiratory distress time (DT).
Abbreviations: IQR, interquartile range; n, number; %, percentage; mm3, microliter; ng, nanogram; ml, milliliter; U/l unites per liter; mg, milligram; dl, deciliter; LDH, Lactate dehydrogenase; CRP, C-reactive protein; pH, measure of hydrogen ion concentration; mmHg, millimeters of mercury; PaCO2, partial pressure of carbon dioxide in the arterial blood; PaO2, partial pressure of oxygen in the arterial blood; PaO2/FIO2 ratio, was defined as the ratio of the partial pressure of arterial oxygen to the percentage of inspired oxygen; ICU, intensive care unit; MV, mechanical ventilation; NIV, non-invasive ventilation.
The number of patients requiring invasive MV was lower (p < 0.001) in the shortest DT tertile than in the remaining two tertiles: 17 (29.8%) in the shortest, 29 (50.9%) in the intermediate and 40 (70.2%) in the longest (Table 1). Using tertiles distribution, the unadjusted analysis showed an increased risk of invasive MV in patients belonging to the longest DT tertile (OR 5.5 [CI 2.48–12.35], p = 0.003). Multivariable regression analysis adjusted for age, comorbidities, total lymphocyte count, platelet count, C-reactive protein and lactate dehydrogenase levels at respiratory distress occurrence confirmed this association (OR 10.7 [CI 2.89–39.4], p < 0.001, Hosmer-Lemeshow test p = 0.013). Clinical outcomes of mortality and hospital stay showed no difference between DT tertiles (Table 1).
DiscussionAlbeit preliminary and retrospective, our multicentre analysis suggested that in hypoxic patients with COVID-19 pneumonia who require MV during their hospital stay, the DT may be associated with the progression of respiratory support. Data showed that the longer the DT, the greater the need for invasive MV, whereas mortality and hospital stay were not affected.
As recently postulated in non-COVID acute respiratory distress syndrome, intense and prolonged inspiratory efforts, generating a high negative intrathoracic pressure, could aggravate lung damage due to the formation of interstitial oedema which, in turn, contributes to increased weight and lung elastance.4,5 This could be particularly relevant in respiratory diseases with a high inflammatory burden, such as severe COVID-19.6 In parallel with our study, a recent study by Menga et al. demonstrated that in hypoxemic COVID-19 patients, moderate-to-severe dyspnoea could be a simple indicator for identifying patients with the highest risk of endotracheal intubation.7 Regardless of severity, our data showed that the long unresolved duration of respiratory distress before the application of positive pressure ventilation may also represent an earlier marker for invasive MV need. Therefore, from a clinical point of view, it may be advisable to apply positive pressure ventilation (even non-invasive) at the first signs of respiratory distress; thus, preventing disease progression. Recommendations on the initial ventilatory approach in patients with COVID-19 with respiratory distress are lacking; however, we believe it was reasonable to consider the use of non-invasive MV as a first option to limit inspiratory effort, and then, monitoring the effect closely, to upgrade to invasive support in case of respiratory distress relief failure.
Our analysis demonstrated limitations and results must be considered with caution. First, retrospective observational design may have introduced unmeasurable biases in patient selection. Likewise, DT onset identification was not rigorous; however, the warning signs for the onset of patient respiratory distress were the focus of a well-structured and shared COVID-19 emergency protocol; therefore, we believe that the DT data were collected with reasonable accuracy. Third, we did not measure oesophageal pressure and, therefore, it is not possible to confirm whether the pathophysiological conditions of persistent negative intrathoracic pressure existed.8
In conclusion, supporting the idea that increased work of breathing can lead to worsening of lung injury,6,7 our preliminary and retrospective data demonstrated that DT was independently associated with the progression of respiratory support in patients with SARS-CoV-2 pneumonia. So, specific strategies may be directed towards identifying and managing early symptoms of respiratory distress, regardless of the levels of hypoxemia and the severity of the dyspnoea itself.
DeclarationsEthics approval and consent to participate: our study was approved by our Ethics Committee of Area Vasta Nord Emilia-Romagna, while consent to the treatment and the participation to the study was collected by telephone from the closest relative for quarantine-related precautions.
Availability of data and materials: the datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interestsThe authors declare that they have no competing interests.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.