Acute exacerbation (AE) signifies a crucial predictor for poor outcomes in bronchiectasis.1 The major mechanism of AE in bronchiectasis is neutrophilic inflammation.2 Consequently, modulating this pathway is crucial in controlling AE.2
Myeloperoxidase (MPO) is the most abundant protein in neutrophils, serving as a hallmark of neutrophil activation in respiratory bursts. Although neutrophil activation protects against microorganisms, exaggerated inflammation can damage alveolar cells.3 During extensive inflammation, neutrophils undergo secondary necrosis, releasing MPO that may damage resident lung cells. In chronic obstructive pulmonary disease, MPO plays a vital role in developing chronic inflammation,3 and its activity is linked to AE.4 However, the association between MPO concentrations and AE status in bronchiectasis remains unclear. Therefore, we aimed to determine whether sputum MPO concentrations reflect stable or AE status in bronchiectasis.
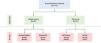
We prospectively enrolled 72 patients with bronchiectasis. All participants underwent sputum examination at the time of study enrolment. Generally, patients in the stable group were followed up after 3 months, and those in the AE group were followed up after 1 to 3 weeks. The patient's condition was determined again at the second visit, and a sputum examination was performed. Patients were classified into four groups according to the absence or presence of AE (stable vs. AE) at the first and second visits (Fig. 1A). Among patients in the stable group at the first visit, patients who had a stable and AE status at the second visit were classified into the stable/stable and stable/AE groups, respectively. Similarly, among patients in the AE group at the first visit, patients who had a stable and AE status at the second visit were classified into the AE/stable and AE/AE groups, respectively. AE of bronchiectasis was defined as the worsening of ≥3 major symptoms lasting ≥48hr, necessitating treatment change. The FACED and Bronchiectasis Severity Index (BSI) scores were used to assess bronchiectasis severity.5 The Sputum MPO function profile was determined by AnyLab F Myeloperoxidase (Z-Biotech, Korea), a point-of-care test (POCT) method based on immunofluorescence. Using a pipette, we added 3–5 μL of phlegm obtained from the patient to the tube containing the detection buffer. After mixing the patient's sputum with the detection buffer, 100 μL of the mixture was distributed into the sample well of the test cartridge and left at room temperature for 15 min. The results were obtained using the measuring device AnyLab F1.
Study population.
At the time of the initial visit, 3 patients in the stable group and 1 patient in the AE group did not visit the hospital at the second visit or the amount of sputum was small, and thus, sputum MPO concentrations could not be measured.
Abbreviations: AE, acute exacerbation.
The baseline characteristics of patients are shown in Table 1. Baseline MPO concentrations were higher in the AE group than in the stable group (median 195.8 ng/mL [interquartile range (IQR) 107.7–832.7] vs. 33.0 ng/mL [IQR 8.2–131.1], p < 0.001) (Fig. 1B). In the stable/stable group, there was no significant change in MPO concentrations between the first and second measurements (median 30.8 ng/mL [IQR 6.4–218.0] vs. 49.9 ng/mL [IQR 15.8–231.7], p = 0.898). Conversely, in the stable/AE group, the second MPO concentration increased compared to the first MPO concentration (median 741.7 ng/mL [IQR 108.4–1194.3] vs. 42.6 ng/mL [IQR 22.4–59.0]) although it was not statistically significant (Fig. 1C). In the AE/stable group, the second MPO concentration decreased compared to the first MPO concentration (median 75.0 ng/mL [IQR 26.1–522.7] vs. 261.5 ng/mL [IQR 127.7–1042.9], p = 0.026). Conversely, there was no significant change in the MPO concentrations in the AE/AE group (median 306.1 ng/mL [IQR 89.9–450.0] vs. 890.3 ng/mL [IQR 517.0–818.5], p = 0.447) (Fig. 1C). As shown in Fig. 1D, there were no statistically significant correlations between MPO concentrations and the BSI and FACED scores in the stable group.
Baseline characteristics.
Data are presented as medians (interquartile ranges) or numbers (%).
Abbreviations: AE, acute exacerbation; MPO, myeloperoxidase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NTM-PD, non-tuberculous mycobacterial pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; mMRC, modified Medical Research Council; FACED, forced expiratory volume in 1 s (F), age (A), presence of chronic colonization by Pseudomonas aeruginosa (C), radiological extension (number of pulmonary lobes affected) (E), dyspnoea (D); BSI, Bronchiectasis Severity Index; WBC, white blood cell; hs-CRP, high-sensitivity C-reactive protein.
Changes in MPO concentrations according to AE status of bronchiectasis.
(a) Changes in MPO concentrations in the stable/stable group
(b) Changes in MPO concentrations in the stable/AE group
(c) Changes in MPO concentrations in the AE/stable group
(d) Changes in MPO concentrations in the AE/AE group
Patients were classified into four groups according to the absence or presence of AE at the first and second visits as follows: stable/stable, stable/AE, AE/stable, and AE/AE groups.
Abbreviations: AE, acute exacerbation; MPO, myeloperoxidase.
Correlation between the disease severity of bronchiectasis and MPO concentrations in the stable group.
Abbreviations: MPO, myeloperoxidase; BSI, bronchiectasis severity index; FACED, forced expiratory volume in 1 s (F), age (A), presence of chronic colonization by Pseudomonas aeruginosa (C), radiological extension (number of pulmonary lobes affected) (E), and dyspnoea (D).
To the best of our knowledge, this is the first study to investigate whether changes in sputum MPO concentrations can reflect AE or stable status of bronchiectasis. In our study, the sputum MPO concentration was a biomarker related to AE status rather than disease severity. We observed no correlation between sputum MPO concentrations and BSI and FACED scores. Conversely, sputum MPO concentrations were significantly correlated with AE status.
Our study has several clinical implications. First, since we measured sputum MPO concentrations using the POCT method, our findings suggest that sputum MPO concentrations can be used as a POCT biomarker for evaluating the development of and recovery from AE in bronchiectasis. Utilising this method, clinicians can determine AE more objectively compared to the current symptoms-based diagnosis of AE. Second, MPO-guided assessment of AE of bronchiectasis may guide antibiotic prescriptions, potentially reducing unnecessary antibiotic use. Third, MPO could be a novel neutrophil-modulating therapeutic target for the treatment of AE in bronchiectasis.
This study has certain limitations. First, it was a single-centre study with a small sample size. Second, we quantitatively measured sputum MPO concentrations but did not evaluate sputum MPO activity. Thus, future studies are necessary regarding MPO activity and AE in bronchiectasis.
In conclusion, there was a significant correlation between changes in AE status and MPO concentrations. Our study suggests that MPO concentrations could be a useful biomarker that reflects AE in bronchiectasis.
FundingThis work was supported by a grant from the National Research Foundation (NRF) of Korea (2020R1A5A2017476). This work was also supported by the NRF grant funded by the Korea government (MSIT) (No.2022R1F1A1074749 to B.Y.) and the research grant of the Chungbuk National University Hospital in 2021.
Artificial intelligence program was not used while writing this manuscript. We thank Sokje Lee, the head of Z-biotech companies, for their assistance with the research.