Methamphetamine causes considerable short- and long-term adverse health effects. Our aim was to assess the effects of methamphetamine use on pulmonary hypertension and lung diseases at the population level.
MethodsThis population-based retrospective study used data from the Taiwan National Health Insurance Research Database between 2000 and 2018 that included 18,118 individuals with methamphetamine use disorder (MUD) and 90,590 matched participants of the same age and sex without substance use disorder as the non-exposed group. A conditional logistic regression model was used to estimate associations of methamphetamine use with pulmonary hypertension and lung diseases such as lung abscess, empyema, pneumonia, emphysema, pleurisy, pneumothorax, or pulmonary hemorrhage. Incidence rate ratios (IRRs) of pulmonary hypertension and hospitalization due to lung diseases were determined between the methamphetamine group and non-methamphetamine group using negative binomial regression models.
ResultsDuring an 8-year observation period, 32 (0.2%) individuals with MUD and 66 (0.1%) non-methamphetamine participants suffered from pulmonary hypertension, and 2652 (14.6%) individuals with MUD and 6157 (6.8%) non-methamphetamine participants suffered from lung diseases. After adjusting for demographic characteristics and comorbidities, individuals with MUD were 1.78 times (95% confidence interval (CI) = 1.07–2.95) more likely to have pulmonary hypertension and 1.98 times (95% CI = 1.88–2.08) more likely to have a lung disease, especially emphysema, lung abscess, and pneumonia in descending order. Furthermore, compared to the non-methamphetamine group, the methamphetamine group was associated with higher risks of hospitalization caused by pulmonary hypertension and lung diseases. The respective IRRs were 2.79 and 1.67. Individuals with polysubstance use disorder were associated with higher risks of empyema, lung abscess, and pneumonia compared to individuals with MUD alone, with respective adjusted odds ratios of 2.96, 2.21, and 1.67. However, pulmonary hypertension and emphysema did not differ significantly between MUD individuals with or without polysubstance use disorder.
ConclusionsIndividuals with MUD were associated with higher risks of pulmonary hypertension and lung diseases. Clinicians need to ensure that a methamphetamine exposure history is obtained as part of the workup for these pulmonary diseases and provide timely management for this contributing factor.
Methamphetamine remains a widely used illicit drug, and its use continues to grow around the world.1 According to the 2017 National Survey on Drug Use and Health, approximately 0.6% of the global population (1.6 million people) reported using methamphetamine in the past year, and 774,000 (0.3%) reported using it in the past month.1 Methamphetamine is known to be associated with adverse public health consequences and increased morbidity2 and mortality.3 In addition to general health impacts, methamphetamine-related adverse effects and toxicities have been documented in cardiovascular, pulmonary, gastrointestinal, dermatological, genitourinary, and neurological organs.4
Methamphetamine is one of the most toxic drugs of abuse, and the high accumulation of methamphetamine in the lungs is likely to render this organ vulnerable to injury.5,6 Methamphetamine was reported to be associated with pulmonary arterial hypertension (PAH)7-9 and other lung diseases,10,11 including pulmonary edema,12,13 pneumonia,14 and emphysema12 in case reports,8,14-16 animal studies,13,17-20 and autopsy studies.12 The association between methamphetamine use and PAH was first reported by Schaiberger et al.15 and was further supported by a retrospective study by Chin et al.21 They found significantly higher rates of methamphetamine use in individuals diagnosed with idiopathic PAH compared with other PAH groups. However, those studies were either case reports or had small sample sizes that would limit their application to other populations. Therefore, extensive epidemiological and comprehensive surveys are needed to investigate the associations of methamphetamine use with pulmonary hypertension and other lung diseases.
We used nationwide population-based data to investigate associations and relative risks of pulmonary hypertension and lung diseases associated individuals with methamphetamine use disorder (MUD) compared to individuals without MUD. The database provided a large sample of participants with an extended study period for a more-comprehensive analysis than previous studies.
Methods and materialsData sourceWe conducted a population-based retrospective study using Taiwan National Health Insurance Research Database (NHIRD) from 2000 to 2018. The NHIRD is derived from Taiwan's single-payer mandatory enrollment National Health Insurance (NHI) Program, which covers up to 99% of the 23 million Taiwanese population.22 The NHIRD includes all medical claims data on disease diagnoses, procedures, drug prescriptions, demographics, and enrollment profiles of all NHI beneficiaries.23
Study sampleIn this retrospective study, we enrolled individuals with newly diagnosed MUD between January 1, 2000 and December 31, 2017 and followed these individuals until December 31, 2018. We defined MUD using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 304.4x and 305.7x and ICD-10 codes F15.1x, F15.2x, and F15.9x. MUD was diagnosed by physicians through self-reports and urine drug tests and then registered in the NHI system. The date of enrollment was defined as the date of the first diagnosis of MUD in the dataset. We also matched individuals of the same age and sex but without MUD or other substance use disorder as the non-exposed group in a 1 to 5 ratio for comparison. Totals of 18,118 individuals with MUD (methamphetamine group) and 90,590 individuals without MUD or other substance use disorder (non-methamphetamine group) were included in our study.
Comorbidities of other substance use disorders that identified during the study period were opioid dependence and abuse (ICD-9-CM codes 304.0x and 305.5x and ICD-10 codes F11.1x and F11.2x), sedative, hypnotic, or anxiolytic dependence and abuse (ICD-9-CM codes 304.1x and 305.4x and ICD-10 codes F13.1x and F13.2x), cocaine dependence and abuse (ICD-9-CM codes 304.2x and 305.6x and ICD-10 codes F14.1x and F14.2x), cannabis dependence and abuse (ICD-9-CM codes 304.3x and 305.2x and ICD-10 codes F12.1x and F12.2x), and hallucinogen dependence and abuse (ICD-9-CM codes 304.6x and 305.3x and ICD-10 codes F16.1x and F16.2x). To clarify the influence of polysubstance use, the 18,118 individuals with MUD were further divided into a MUD-only group and a polysubstance group for analysis.
Outcomes of interestThe outcomes of interest were pulmonary hypertension (ICD-9-CM code 416 and ICD-10 codes I27.x), lung abscess (ICD-9-CM code 513 and ICD-10 codes J85.x), empyema (ICD-9-CM code 510 and ICD-10 codes J86.x), pneumonia (ICD-9-CM codes 480∼486 and ICD-10 codes J13.x∼J18.x), emphysema (ICD-9-CM code 492 and ICD-10 codes J43.x), pleurisy (ICD-9-CM code 511 and ICD-10 codes J90.x), pneumothorax (ICD-9-CM code 512 and ICD-10 codes J93.x), pulmonary hemorrhage (ICD-9-CM code 770 and ICD-10 codes J94.x), and composite lung disease. Composite lung disease was defined as the presence of one or more diagnoses such as lung abscess, empyema, pneumonia, emphysema, pleurisy, pneumothorax, or pulmonary hemorrhage. We observed the occurrence of the above conditions within the period of 3 years prior to and 5 years after a MUD diagnosis because most individuals already showed patterns of methamphetamine abuse or dependence before their first MUD diagnosis was made.24,25 Therefore, we considered our outcomes prior- and post-MUD diagnosis.
CovariatesCharlson's comorbidity index (CCI) was abstracted from NHIRD claims 1 year before the MUD diagnosis as covariates to assess the burden of comorbidities.26 We excluded chronic pulmonary disease diagnoses from the CCI because they were part of our outcomes of interest. Finally, we divided individuals according to CCI scores into four groups: 0, 1, 2, and ≥3 for adjustment. We also obtained data of the following comorbidities: myocardial infarction, congestive heart failure, peripheral vascular disease, cardiovascular disease, diabetes mellitus (DM), and human immunodeficiency virus (HIV) because these diseases were reported to be associated with pulmonary hypertension in previous studies.27-29
Statistical analysisDescriptive statistics were used to summarize participant characteristics. Independent t-test and Chi-squared test were respectively adopted for continuous and categorical variables. For the analysis of the methamphetamine versus non-methamphetamine groups, we used conditional logistic regression models to estimate the risk of pulmonary hypertension, composite lung disease, and each lung disease. Incidence rate ratios (IRRs) of hospitalization for pulmonary hypertension and composite lung disease between these two groups were also estimated using a negative binomial regression model by counting hospitalization events and person-times of follow-up. All conditional logistic regression models and negative binomial regression models were adjusted for variables such as the age at enrollment, the comorbidity of other substance use disorders (yes/no), and the CCI score. These models did not include sex for adjustment because this variable was already matched in the matching processes.
To clarify the influence of polysubstance use, all individuals with MUD were further divided into a MUD-only group and a polysubstance group for analysis. The logistic regression models were applied to estimate the risk of pulmonary hypertension, composite lung disease, and each lung disease between these two groups. Because significant differences in these two groups at the baseline limited proper matching, variables such as the age at enrollment, the CCI score, and sex were adjusted for in the logistic regression models.
All statistical analyzes were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). This study was reviewed and approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB no. N201903151).
Sensitivity analysisPrevious studies demonstrated that HIV30 and other substance use31 were also associated with lung injury. In order to more specifically examine the adverse effects of methamphetamine use on lung, we conducted two sensitivity analyses that excluded participants with HIV or other substance use disorders for further analysis.
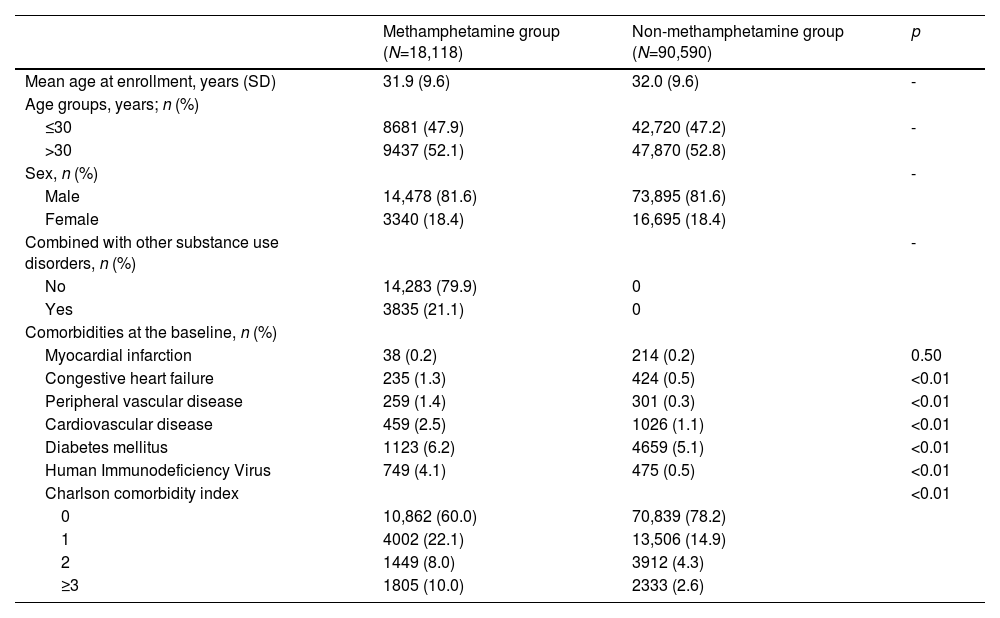
ResultsDuring an 8-year observation period, totals of 18,118 individuals in the methamphetamine group and 90,590 individuals of the same age and sex in the non-methamphetamine group were included in the analysis (Table 1). The mean age was 32 (standard deviation [SD]: 9.7) years, with 81.6% male individuals. The methamphetamine group had a higher percentage of individuals with CCI scores of ≥1 than did the non-methamphetamine group. They were also more likely to have congestive heart failure, peripheral vascular disease, cardiovascular disease, DM, and HIV than those in the non-methamphetamine group. Approximately 21.1% of MUD individuals also had other substance use disorders.
Comparisons of characteristics at the date of first methamphetamine use disorder diagnosis between the methamphetamine group and non-methamphetamine group (N=108,708).
Abbreviation: SD, standard deviation.
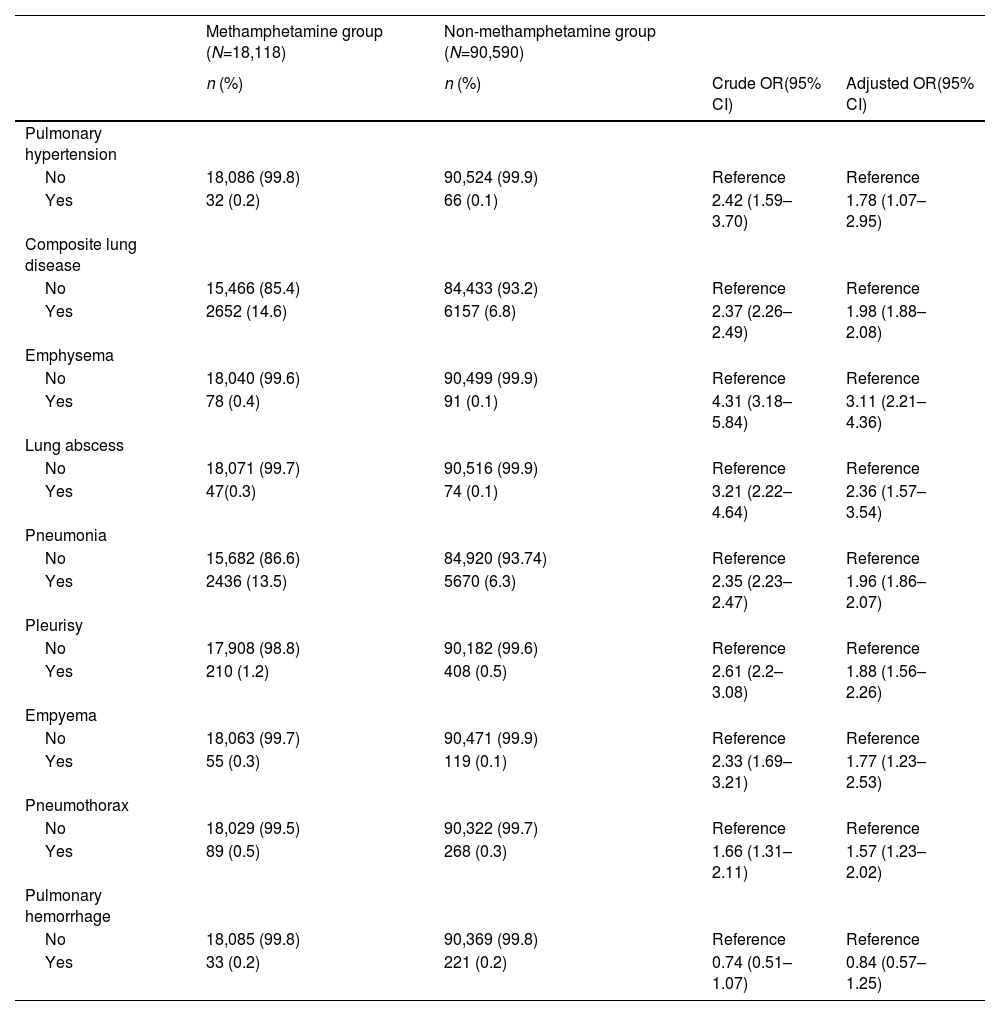
In the methamphetamine group, 0.2% of the MUD individuals had pulmonary hypertension and 14.6% of the MUD individuals had composite lung disease. Meanwhile, 0.1% of the participants in the non-methamphetamine group had pulmonary hypertension and 6.8% of the participants had composite lung disease in the non-methamphetamine group (Table 2). Among MUD individuals with composite lung diseases, 91.8% of them had only one lung disease, 8.0% of them had two or three lung diseases, and 0.2% of them had four lung diseases. However, among non-methamphetamine individuals with composite lung diseases, 93.7% of them had only one lung disease, 6.1% of them had two or three lung diseases, and 0.2% of them had four lung diseases. In the multivariate conditional logistic regression, the methamphetamine group showed higher risks of pulmonary hypertension and composite lung disease compared to the non-methamphetamine group with adjusted odds ratios (aORs) of 1.78 (95% confidence interval [CI] = 1.07–2.95) and 1.98 (95% CI: 1.98–2.08) respectively. Additionally, individuals in the methamphetamine group had a 3.11-fold (95% CI = 4.01–41.27) risk of emphysema, a 2.36-fold (95% CI = 1.82–4.44) of lung abscess, a 1.96-fold (95% CI = 1.86–2.07) risk of pneumonia, a 1.88-fold (95% CI = 1.56–2.26) risk of pleurisy, a 1.77-fold (95% CI = 1.23–2.53) risk of empyema, and a 1.57-fold (95% CI = 1.23–2.02) risk of pneumothorax compared to the non-methamphetamine group. However, the risk of pulmonary hemorrhage did not significantly differ between these two groups.
Crude and adjusted odds ratios (ORs) of pulmonary hypertension and lung disease between the methamphetamine and non-methamphetamine groups (N=108,708).
Composite lung disease was defined as the presence of one or more diagnoses such as lung abscess, empyema, pneumonia, emphysema, pleurisy, pneumothorax, or pulmonary hemorrhage.
Adjusted ORs were estimated from the conditional logistics regression adjusted for age at enrollment, combined with other substance use disorders (yes/no) and Charlson comorbidity index scores.
Abbreviation: CI, confidence interval.
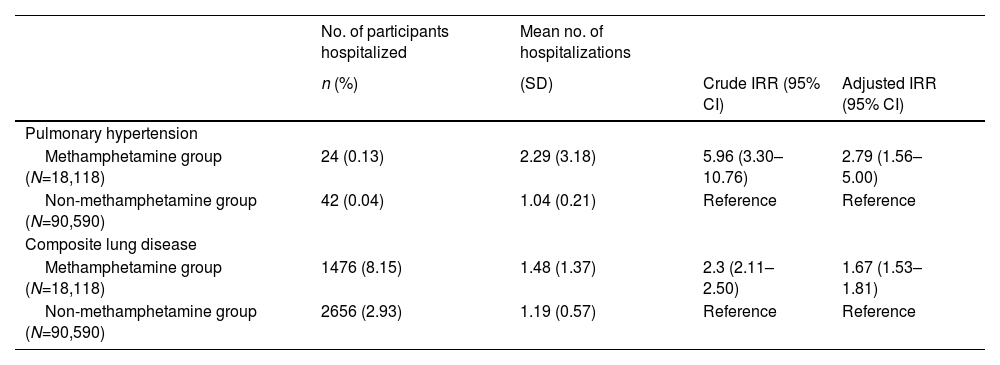
Individuals in the methamphetamine group had higher hospitalization rates for pulmonary hypertension and composite lung disease than their counterparts in the non-methamphetamine group during the 8-year observation period (Table 3). The average number of hospitalizations for each individual was also higher in the methamphetamine group. The average number of hospitalizations for pulmonary hypertension was 2.29 in the methamphetamine group and 1.04 in the non-methamphetamine group. Results of the negative binomial regression showed that the methamphetamine group exhibited a 279% higher risk of hospitalization for pulmonary hypertension than the non-methamphetamine group (incidence rate ratio [IRR] = 2.79, 95% CI = 1.56–5.00).
Incidence rate ratios (IRRs) of hospitalization for pulmonary hypertension and composite lung disease between the methamphetamine and non-methamphetamine groups (N=108,708).
The adjusted IRR used a conditional negative binomial regression model adjusted for age at enrollment, combined with other substance use disorders (yes/no), and the Charlson comorbidity index score.
Composite lung disease was defined as the presence of one or more diagnoses such as lung abscess, empyema, pneumonia, emphysema, pleurisy, pneumothorax, or pulmonary hemorrhage.
Abbreviation: CI, confidence interval; no., number; SD, standard deviation.
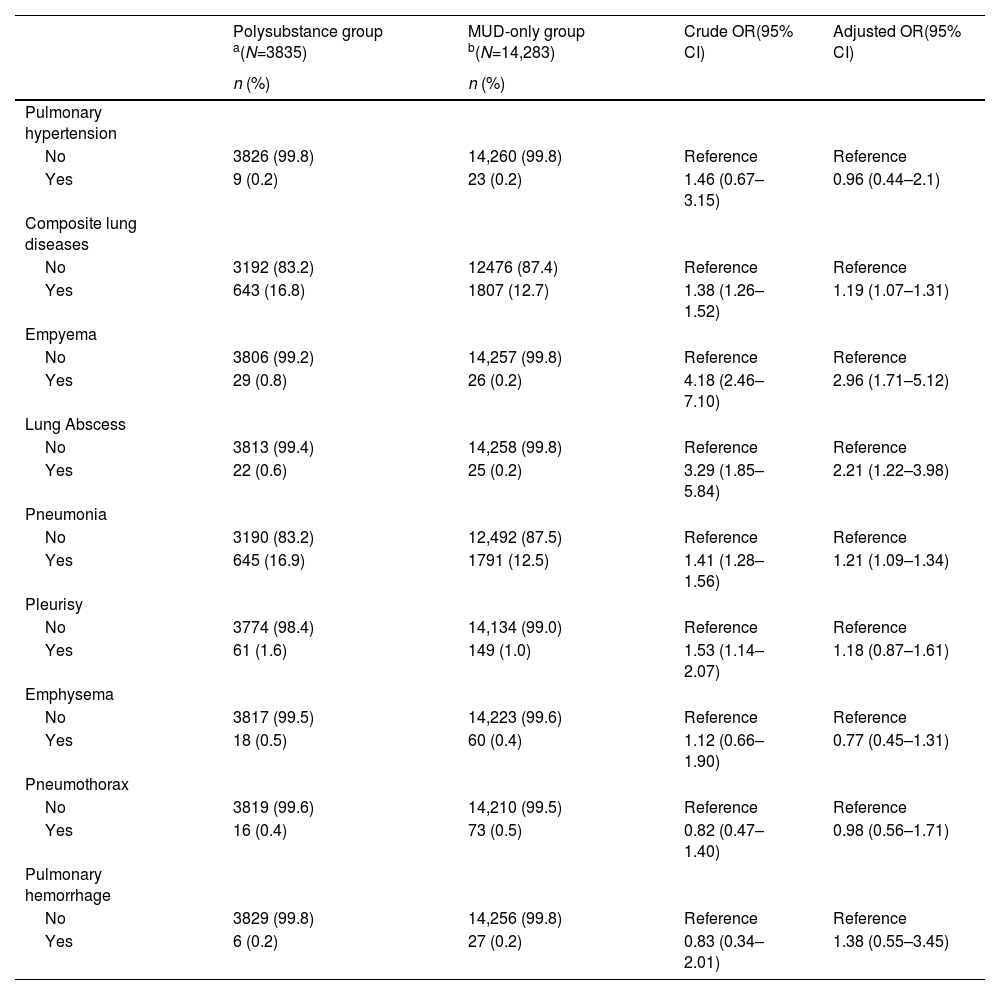
We also compared the risks of these lung diseases between individuals with MUD only to those comorbid MUD with other substance use disorder. Table A.1 shows that polysubstance group were older and had a higher percentage with peripheral vascular disease, DM, HIV, and CCI scores of ≥1 compared to the MUD-only group. The polysubstance group also had higher risks of empyema, lung abscess, and pneumonia than the MUD-only group. However no significant difference of the risk of pulmonary hypertension, emphysema, or pneumothorax were observed (Table 4).
Crude and adjusted odds ratios (ORs) of pulmonary hypertension and lung diseases among methamphetamine use disorder (MUD) individuals with and those without other substance use disorders (N=18,118).
| Polysubstance group a(N=3835) | MUD-only group b(N=14,283) | Crude OR(95% CI) | Adjusted OR(95% CI) | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Pulmonary hypertension | ||||
| No | 3826 (99.8) | 14,260 (99.8) | Reference | Reference |
| Yes | 9 (0.2) | 23 (0.2) | 1.46 (0.67–3.15) | 0.96 (0.44–2.1) |
| Composite lung diseases | ||||
| No | 3192 (83.2) | 12476 (87.4) | Reference | Reference |
| Yes | 643 (16.8) | 1807 (12.7) | 1.38 (1.26–1.52) | 1.19 (1.07–1.31) |
| Empyema | ||||
| No | 3806 (99.2) | 14,257 (99.8) | Reference | Reference |
| Yes | 29 (0.8) | 26 (0.2) | 4.18 (2.46–7.10) | 2.96 (1.71–5.12) |
| Lung Abscess | ||||
| No | 3813 (99.4) | 14,258 (99.8) | Reference | Reference |
| Yes | 22 (0.6) | 25 (0.2) | 3.29 (1.85–5.84) | 2.21 (1.22–3.98) |
| Pneumonia | ||||
| No | 3190 (83.2) | 12,492 (87.5) | Reference | Reference |
| Yes | 645 (16.9) | 1791 (12.5) | 1.41 (1.28–1.56) | 1.21 (1.09–1.34) |
| Pleurisy | ||||
| No | 3774 (98.4) | 14,134 (99.0) | Reference | Reference |
| Yes | 61 (1.6) | 149 (1.0) | 1.53 (1.14–2.07) | 1.18 (0.87–1.61) |
| Emphysema | ||||
| No | 3817 (99.5) | 14,223 (99.6) | Reference | Reference |
| Yes | 18 (0.5) | 60 (0.4) | 1.12 (0.66–1.90) | 0.77 (0.45–1.31) |
| Pneumothorax | ||||
| No | 3819 (99.6) | 14,210 (99.5) | Reference | Reference |
| Yes | 16 (0.4) | 73 (0.5) | 0.82 (0.47–1.40) | 0.98 (0.56–1.71) |
| Pulmonary hemorrhage | ||||
| No | 3829 (99.8) | 14,256 (99.8) | Reference | Reference |
| Yes | 6 (0.2) | 27 (0.2) | 0.83 (0.34–2.01) | 1.38 (0.55–3.45) |
Composite lung disease was defined as the presence of one or more diagnoses such as lung abscess, empyema, pneumonia, emphysema, pleurisy, pneumothorax or pulmonary hemorrhage.
Table A.2 shows the results of the sensitivity analysis which excluded individuals with other substance use disorders or HIV. The methamphetamine group still had an increased risk for pulmonary hypertension and composite lung disease than the non-methamphetamine group in the sensitivity analysis.
DiscussionIn this population-based study of a 8-year observation period, we observed that individuals with MUD were associated with higher risks of pulmonary hypertension and lung diseases. The three major lung diseases in terms of increased risks were emphysema, lung abscess, and pneumonia in descending order. These individuals also demonstrated increased risks of hospitalization for these conditions which might reflect the disease severity. Our results showed that methamphetamine use was probably an important contributor to pulmonary hypertension, emphysema, and pulmonary infections, and these findings are important in clinical practice for enhancing management of these diseases.
The development of pulmonary hypertension is associated with clinical deterioration and a substantially increased mortality risk.32 Prolonged methamphetamine abuse can result in markedly increased susceptibility to pulmonary hypertension21,33 and is recognized as definitely being associated with PAH.34 Our population-based study confirmed this association and showed a 78% increased risk of pulmonary hypertension and a more than twofold risk of hospitalization for pulmonary hypertension in individuals with MUD. To the best of our knowledge, this is probably the first large observational study to investigate the associations between pulmonary hypertension and individuals with MUD.
Although the actual underlying mechanisms of methamphetamine-induced pulmonary hypertension are still unclear, several hypotheses have been proposed. Volkow's study showed that methamphetamine concentrations were high in lung tissues, and the lungs demonstrated much higher methamphetamine uptake, faster peak times, and slower clearance than other organs.6 Methamphetamine is a substrate that acts on norepinephrine, dopamine, and serotonin transporter with vasoconstrictive and growth-modulating effects in smooth muscle cells. These transporters are observed in the pulmonary vasculature, which suggests a possible involvement of methamphetamine in the development of PAH.35-37 Clinical features of methamphetamine-induced pulmonary hypertension were reported to differ from those of idiopathic PAH.38 and methamphetamine-induced pulmonary hypertension presents less-favorable hemodynamics which suggests concomitant myocardial toxicity from methamphetamine use. Therefore, assessment of the methamphetamine exposure history is suggested when evaluating individuals with pulmonary hypertension for timely management of this risk factor.38
In our study, individuals with MUD were almost twice as likely to have a lung disease, especially emphysema, lung abscess, and pneumonia in descending order. The relationship between methamphetamine use and barotrauma injuries such as emphysema and pneumothorax were reported in case studies.39-41 Our study showed that methamphetamine use was strongly associated with emphysema and pneumothorax which were consistent with previous studies. A previous study showed that inhalation was a key way for early-onset emphysema in heroin users.42 In Taiwan, most individuals with MUD used methamphetamine by smoking or inhaling the vapor. Therefore, methamphetamine use, especially the inhalation form of use, may also be an important contributor to pneumothorax and emphysema as we observed in this study.
Our study also demonstrated that methamphetamine use was associated with lung infections such as lung abscess, empyema, pneumonia, and pleurisy. The effects of methamphetamine on adaptive immunity defenses have not yet been extensively described, and the mechanism remained largely unknown.43 HIV and polysubstance abuse are risk factors for lung abscess and pneumonia according to previous studies.44,45 In our study, methamphetamine users had a higher percentage of comorbidities at the baseline, especially of HIV, than non-methamphetamine users. In order to more specifically examine the adverse effects of methamphetamine use on lung infections, we conducted two sensitivity analyses that excluded participants with HIV or polysubstance abuse. The methamphetamine group still had an increased risk for lung infections which implied that methamphetamine use itself might also be a contributor to lung infections.
However, it is also possible that these associations with composite lung disease were partly due to the presence of several important confounders related to methamphetamine use, such as cigarette smoking, exposure to other harmful substances, and lifestyles2,46-48 rather than the direct pharmacological effects of methamphetamine. In our previous study, we found an extremely high rate of cigarette smoking (93.1%) among MUD individuals.49 So these associations with composite lung disease might also be viewed as a mixed consequence of methamphetamine use with these confounders, especially cigarette smoking. Although we could not rule out the impact of these confounders due to the limitations of NHIRD, our results may support the contribution of methamphetamine to higher risks of pulmonary hypertension and selected lung diseases, as observed at population level.
Our results did not find a statistical difference in the rate of pulmonary hemorrhage between the methamphetamine and non-methamphetamine groups, which is inconsistent with previous case reports.50-52 This may partially have been due to the small event number observed in our population. Determining the relationship between methamphetamine use and pulmonary hemorrhage requires further research too.
In summary, our findings support the thesis that methamphetamine use is an important risk factor associated with pulmonary hypertension and lung injury. Careful assessment of the methamphetamine exposure history is suggested with these diseases because of these associations. Once methamphetamine use is confirmed, early counseling or other prevention efforts should be provided to reduce the adverse effects of methamphetamine on pulmonary hypertension and lung injury.
Strengths and limitationsThe main strength of this study was the large nationwide population we surveyed. This advantage allowed us to understand long-term courses of people seeking treatment for MUD in the real world. Several limitations should be addressed. First, individuals with MUD were identified using ICD codes in the NHI system that could only reflect a lifetime history of methamphetamine use and the timing of seeking medical intervention. Cumulative incidences of lung injuries associated with methamphetamine could not be estimated. Second, information on the severity of MUD was not included in the dataset. We could not assess the dose effects of methamphetamine use on lung diseases. Third, MUD was diagnosed by physicians through self-reports and urine drug tests and then registered in the NHIRD. Although social desirability bias cannot be excluded, it was more likely to have occurred in the non-methamphetamine group. Such a misclassification would have attenuated the risk effect if it did exist. The true risk would be higher. Fourth, several confounders related to methamphetamine use were not available in the NHIRD for adjustment. These confounders were cigarette smoking, other exposure to harmful substances, and lifestyles.2,46-49 Finally, we only included individuals with methamphetamine use who sought treatment in Taiwan, which may have limited the generalizability of the study results to MUD individuals in the community or other populations.
ConclusionsIndividuals with MUD are associated with an increased risk of pulmonary hypertension and lung injury. These findings strongly support recommendations of the 2018 World Symposium on Pulmonary Hypertension that methamphetamine is considered a “definite” risk factor.29 Although the mechanisms and causal relationships surrounding these associations need to be clarified by more research, clinicians need to ensure that a methamphetamine exposure history is obtained as part of the workup for these pulmonary diseases and provide timely management for this contributing factor.
AbbreviationsCCI, Charlson's comorbidity index; DM, diabetes mellitus, HIV, human immunodeficiency virus; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; MUD, methamphetamine use disorder; PAH, pulmonary arterial hypertension; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database
Authors’ contributionsDr. Shao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Shao, Fang.
Acquisition, analysis, or interpretation of data: Fang, Huang.
Drafting of the manuscript: Fang, Huang.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Shao, Fang.
Obtained funding: Shao, Huang.
Administrative, technical, or material support: Shao.
Supervision: All authors
Availability of data and materialsData is available from the Health and Welfare Data Center published by the Ministry of Health and Welfare. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the Health and Welfare Data Center Administration (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html).
FundingThis study was supported by grants from the National Science and Technology Council, Taiwan (110-2341-B-715-013) and Bali Psychiatric Center, Taiwan (MOHW#10906). The funders have no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
We are grateful to Health Data Science Center, Ministry of Health and Welfare, Taiwan for providing insurance claims data, administrative and technical support.