In COPD, the bronchial epithelium shows a pathologically activated Wnt pathway. Sclerostin (SOST) is a secreted glycoprotein that is associated with bone metabolism and blocks the Wnt pathway. We hypothesized that low sclerostin levels might be associated with lung function and COPD exacerbations in patients.
MethodsWe studied 139 outpatients with stable COPD and normal kidney function. We assessed the serum levels of SOST and bone metabolism parameters, body composition, clinical characteristics and lung function at baseline. We followed the patients prospectively for 12 months after enrolment. Moderate exacerbations and hospital admissions were recorded during follow-up.
ResultsThe serum SOST levels were 23.98±7.6 pmol/l (men: 25.5±7.7 pmol/l, women: 20.3±5.9 pmol/l (p < 0.001)). SOST showed correlations with age (r = 0.36), FFMI (r = 0.38), FEV1 (r = 0.27), DLCO (r = 0.39), 6MWD (r = 0.19) and CAT (r = -0.24). In multivariate linear regression analysis, only age (beta=0.264) and FFMI (beta=1.241) remained significant. SOST showed a significant negative correlation with serum phosphorus (r = -0.29). Cox proportional risk analysis indicated that patients in the lower tertile of SOST levels were at higher risk of moderate COPD exacerbation (HR 2.015, CI95% 1.136–3.577, p = 0.017) and hospital admission due to COPD (HR 5.142, CI95% 1.380–19.158, p = 0.015) than the rest of the patients.
ConclusionsSOST levels are associated with body composition and lung function in patients with COPD. Furthermore, lower SOST levels predict a higher risk of exacerbations and hospitalization.
COPD is a leading cause of mortality worldwide.1 COPD exacerbations are among the most relevant outcomes in COPD, because it leads to poorer prognosis2 and diminished quality of life.3 Several traits4–6 have been identified as potential triggers of exacerbations, although the only predictor of risk proposed by international guidelines is previous exacerbations7. No serum biomarkers have demonstrated a consistent association with COPD outcomes. Sclerostin (SOST) has been widely studied in the context of bone metabolism, because it is secreted by osteocytes and regulates bone turnover.8–9 SOST binds LRP5/6, a coreceptor of the Wnt canonical pathway, and blocks this signal transduction pathway.8–9 SOST is also secreted by numerous other cell types, including bronchial and skeletal muscle cells, and is considered a systemic hormone.10 In addition, Carlier et al.11 have recently demonstrated that the canonical Wnt pathway is aberrantly activated in the airway epithelium in patients with COPD. The extrinsic activation of this pathway inhibits epithelial differentiation, polarity and barrier function, and induces TGF-beta related epithelial-to-mesenchymal transition.
Furthermore, SOST may play an important role in muscle-bone crosstalk, regulating muscle mass and lung function, and modifying body composition, although not all study findings are concordant.8,9,12–14
To test this hypothesis, we analysed the associations of blood SOST with body composition, bone metabolism, lung function and clinical parameters in a well-characterized cohort of patients with COPD. Importantly, we also prospectively assessed the frequency of exacerbations for 12 months. To the best of our knowledge, no previous studies have evaluated blood levels of SOST in COPD.
MethodsThis was an observational prospective study performed in a COPD outpatient clinic from a third level hospital in Spain from November 2017 to October 2018.
The study was approved by the Ethics Committee of our institution (2017.035). All patients provided written informed consent to participate in this study.
ParticipantsPatients were consecutively recruited during routine visits to the monographic COPD outpatient clinic.
Inclusion criteria: Adult patients with COPD according to GOLD Guidelines7 who were older than 40 years of age.
Exclusion criteria: 1) Patients using treatments interfering with bone resorption (vitamin D metabolites, oral calcium, estrogens, PTH, other antiosteoporotic agents, systemic glucocorticoids, etc.); 2) treatment with pulmonary rehabilitation during the study or 6 months before the inclusion period; 3) COPD exacerbation 8 weeks before the study; 4) other conditions that could alter bone resorption (primary hyperparathyroidism or any other cause of hyper- or hypocalcaemia, recent osteoporotic or traumatic fractures, sarcoidosis, cancer, acute or chronic kidney failure, or rheumatoid arthritis).
MeasurementsThe methodologic aspects of this study are similar to those in a previously published study.15 In summary, trained staff recorded clinical information and performed lung function tests. Spirometry was performed according to the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Guidelines.16 Diffusing capacity of the lung for carbon monoxide (DLCO) was measured in accordance with ATS/ERS Guidelines.17 Six-minute walking tests were performed according to the SEPAR protocol.18 We estimated body composition with a bioelectrical impedance device (OMROM BF511, Omrom, Japan).
Blood samples were collected from all participants after they had signed the consent form. To avoid a possible effect of circadian variability on SOST levels, we obtained all samples between 10:00 and 12:00 pm. Serum total calcium, phosphorus and albumin were measured with a Siemens traceable enzymatic method assay (ADVIA 2400 Analyzer, Siemens Healthcare Diagnostics, Germany). Total calcium levels were corrected with albumin. C-reactive protein (CRP) was measured with immunoturbidimetry (ADVIA 2400 Analyzer, Siemens Healthcare Diagnostics, Germany), and 25(OH)D concentrations were measured with an automated competitive chemiluminescence assay (Liaison XL, DiaSorin Inc, Stillwater MN USA). We measured serum intact PTH (iPTH) levels with the automated chemiluminescence competitive assay IDS iSYS (Immunodiagnostic Systems Ltd, Boldon, UK), P1NP (an established marker of osteoblastic function) and β-Crosslaps (a marker of osteoclastic function) with a specific chemiluminescence immunoassay in iSYS (IDS-iSYS Multi-Discipline Automated Analyser, Pouilly-en Auxois, France). Serum SOST levels were measured with a highly sensitive specific immunoassay (Sclerostin HS ELISA, TECO Medical Sissach Switzerland).
After entering the study, patients were followed up for 12 months. We prospectively recorded moderate COPD exacerbations (exacerbations in patients treated with antibiotics and/or systemic corticosteroids) and hospitalizations due to severe COPD exacerbations, by asking the patients during follow-up visits (6 and 12 months after entering the study) and checking the medical records from the hospital and primary care. General or emergency physicians unaffiliated with this study made the diagnosis of exacerbation or the decision to hospitalize the patients.
Statistical analysisData are presented as mean±SD for normally distributed data or median (interquartile range) for nonparametric data. Sample size calculation was performed in Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.), with an α risk of 0.05 and a β risk of 0.2. Differences between groups were analysed with unpaired t tests for parametric data or Mann-Whitney tests for nonparametric data. Normal distributions were evaluated with Kolmogorov-Smirnov tests. Correlations between data sets were examined with the Pearson (r) correlation coefficient for parametric data or the Spearman rank (rs) correlation coefficient for nonparametric data. Multivariate linear regression was performed to assess factors explaining the variance in SOST. Kaplan-Meier estimates were used to calculate the proportion of participants experiencing an event over time. Univariate and multivariate analyses with Cox proportional risk analysis were performed in SPSS Software version 25.00 for PC to identify risk factors associated with moderate COPD exacerbations and hospitalizations due to COPD exacerbations. Differences were considered significant if p values were less than 0.05. All reported p values are two-sided.
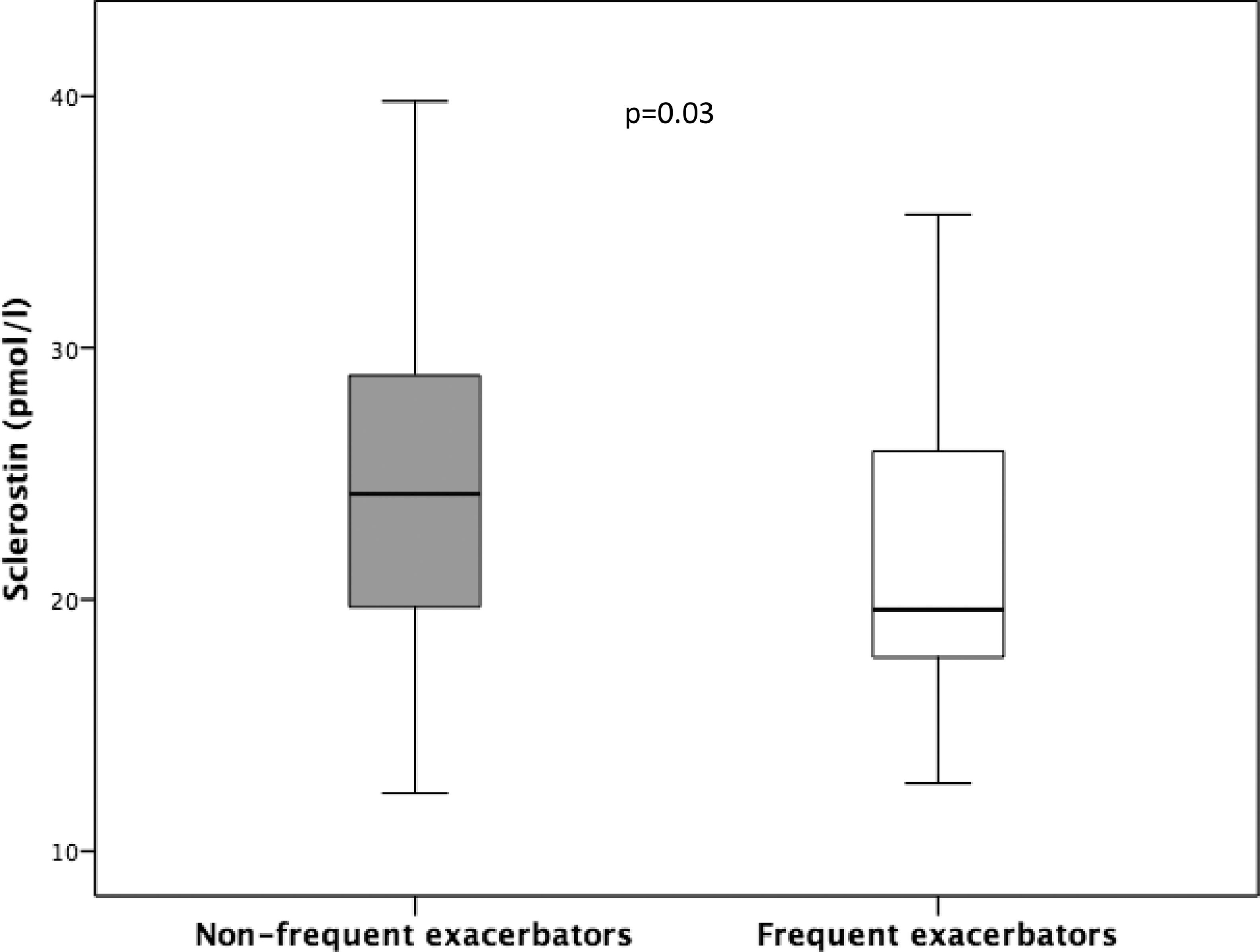
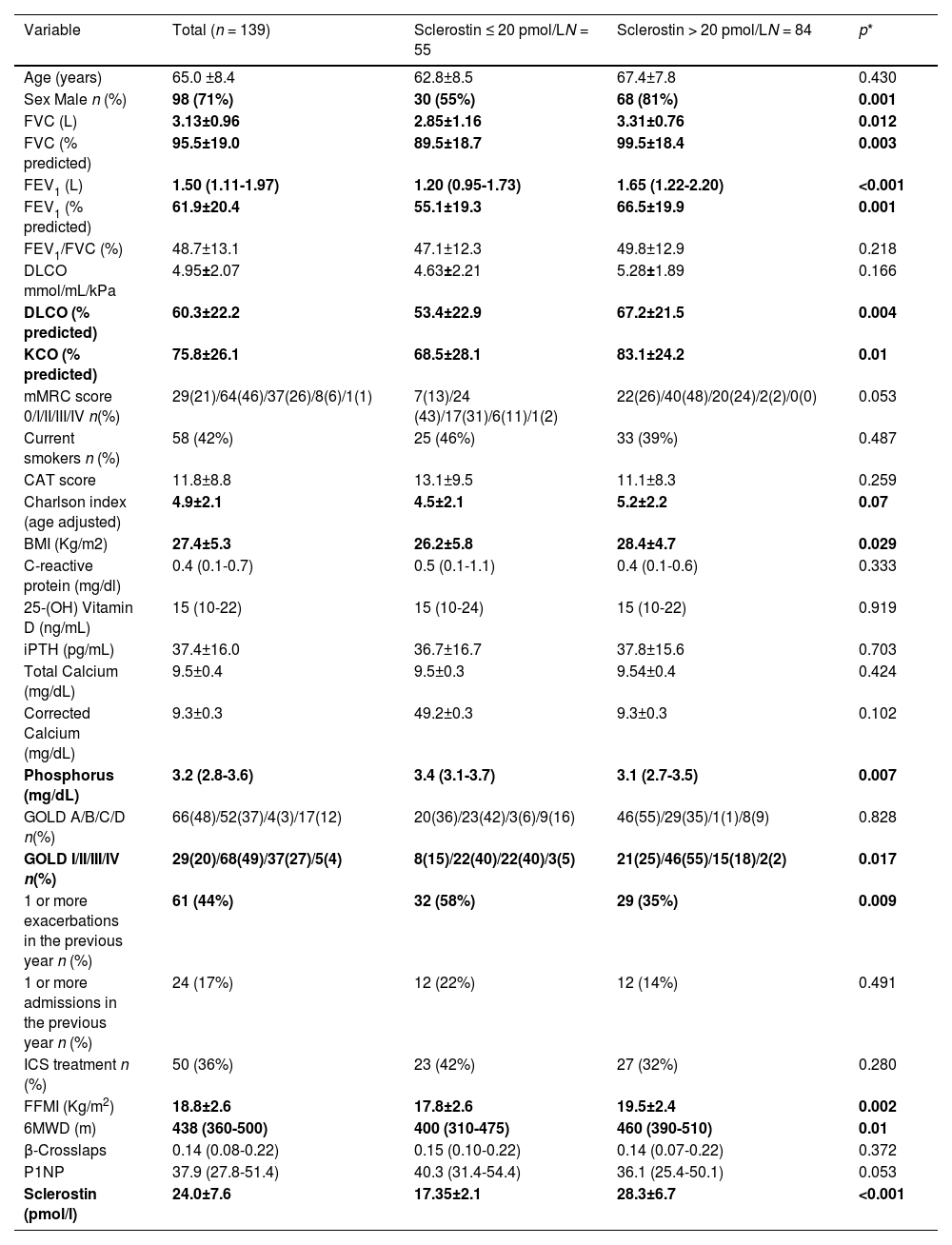
ResultsCharacteristics of the patientsA total of 139 patients were finally included in the study (flowchart for patient selection is shown in Fig. 1). Demographic, clinical and biochemical data are shown in Table 1. The mean patient age was 65±8.4 years, and most were men (70.5%). A high prevalence of current smokers (41.7%) was observed. Most patients had moderate obstruction and a moderate decrease in DLCO. The serum 25(OH)D levels were 15 (10–22) ng/mL, and the serum iPTH levels were 37.3±16 pg/mL. The SOST levels were 23.9±7.6 pmol/l and were higher in men 25.5±7.7 pmol/l than in women 20.3±5.9 pmol/l (p < 0.001). The group of patients with COPD and lower SOST levels included more men, and had worse lung function, less exercise capacity, lower FFMI, a trend towards higher dyspnoea, and higher exacerbation and hospitalization rates. However, the two groups had similar GOLD ABCD distribution, comorbidities and levels of molecules associated with phosphocalcic metabolism, with the exception of phosphorus, which was higher in the group of patients with lower SOST levels. SOST levels varied with GOLD obstruction (GOLD I 27.1±9.2 pmol/l, GOLD II 24.3±7.5 pmol/l, GOLD III 21.5±5.1 pmol/l, GOLD IV 19.5±6.5 pmol/l, p = 0.011) (Fig. 2), and were lower in frequent Vs. non-frequent exacerbators (22.4±7.1 pmol/l vs. 25.2±7.7 pmol/l(p = 0.03) (Fig. 3).
Demographic, clinical and biochemical characteristics of total patients included in the study and patients with lower tertile (33th percentile) vs upper (34th percentile) sclerostin levels.
| Variable | Total (n = 139) | Sclerostin ≤ 20 pmol/LN = 55 | Sclerostin > 20 pmol/LN = 84 | p* |
|---|---|---|---|---|
| Age (years) | 65.0 ±8.4 | 62.8±8.5 | 67.4±7.8 | 0.430 |
| Sex Male n (%) | 98 (71%) | 30 (55%) | 68 (81%) | 0.001 |
| FVC (L) | 3.13±0.96 | 2.85±1.16 | 3.31±0.76 | 0.012 |
| FVC (% predicted) | 95.5±19.0 | 89.5±18.7 | 99.5±18.4 | 0.003 |
| FEV1 (L) | 1.50 (1.11-1.97) | 1.20 (0.95-1.73) | 1.65 (1.22-2.20) | <0.001 |
| FEV1 (% predicted) | 61.9±20.4 | 55.1±19.3 | 66.5±19.9 | 0.001 |
| FEV1/FVC (%) | 48.7±13.1 | 47.1±12.3 | 49.8±12.9 | 0.218 |
| DLCO mmol/mL/kPa | 4.95±2.07 | 4.63±2.21 | 5.28±1.89 | 0.166 |
| DLCO (% predicted) | 60.3±22.2 | 53.4±22.9 | 67.2±21.5 | 0.004 |
| KCO (% predicted) | 75.8±26.1 | 68.5±28.1 | 83.1±24.2 | 0.01 |
| mMRC score 0/I/II/III/IV n(%) | 29(21)/64(46)/37(26)/8(6)/1(1) | 7(13)/24 (43)/17(31)/6(11)/1(2) | 22(26)/40(48)/20(24)/2(2)/0(0) | 0.053 |
| Current smokers n (%) | 58 (42%) | 25 (46%) | 33 (39%) | 0.487 |
| CAT score | 11.8±8.8 | 13.1±9.5 | 11.1±8.3 | 0.259 |
| Charlson index (age adjusted) | 4.9±2.1 | 4.5±2.1 | 5.2±2.2 | 0.07 |
| BMI (Kg/m2) | 27.4±5.3 | 26.2±5.8 | 28.4±4.7 | 0.029 |
| C-reactive protein (mg/dl) | 0.4 (0.1-0.7) | 0.5 (0.1-1.1) | 0.4 (0.1-0.6) | 0.333 |
| 25-(OH) Vitamin D (ng/mL) | 15 (10-22) | 15 (10-24) | 15 (10-22) | 0.919 |
| iPTH (pg/mL) | 37.4±16.0 | 36.7±16.7 | 37.8±15.6 | 0.703 |
| Total Calcium (mg/dL) | 9.5±0.4 | 9.5±0.3 | 9.54±0.4 | 0.424 |
| Corrected Calcium (mg/dL) | 9.3±0.3 | 49.2±0.3 | 9.3±0.3 | 0.102 |
| Phosphorus (mg/dL) | 3.2 (2.8-3.6) | 3.4 (3.1-3.7) | 3.1 (2.7-3.5) | 0.007 |
| GOLD A/B/C/D n(%) | 66(48)/52(37)/4(3)/17(12) | 20(36)/23(42)/3(6)/9(16) | 46(55)/29(35)/1(1)/8(9) | 0.828 |
| GOLD I/II/III/IV n(%) | 29(20)/68(49)/37(27)/5(4) | 8(15)/22(40)/22(40)/3(5) | 21(25)/46(55)/15(18)/2(2) | 0.017 |
| 1 or more exacerbations in the previous year n (%) | 61 (44%) | 32 (58%) | 29 (35%) | 0.009 |
| 1 or more admissions in the previous year n (%) | 24 (17%) | 12 (22%) | 12 (14%) | 0.491 |
| ICS treatment n (%) | 50 (36%) | 23 (42%) | 27 (32%) | 0.280 |
| FFMI (Kg/m2) | 18.8±2.6 | 17.8±2.6 | 19.5±2.4 | 0.002 |
| 6MWD (m) | 438 (360-500) | 400 (310-475) | 460 (390-510) | 0.01 |
| β-Crosslaps | 0.14 (0.08-0.22) | 0.15 (0.10-0.22) | 0.14 (0.07-0.22) | 0.372 |
| P1NP | 37.9 (27.8-51.4) | 40.3 (31.4-54.4) | 36.1 (25.4-50.1) | 0.053 |
| Sclerostin (pmol/l) | 24.0±7.6 | 17.35±2.1 | 28.3±6.7 | <0.001 |
FVC= Forced Vital Capacity, FEV1= Forced expiratory Volume in the first second, mMRC= modified Medical Research Council Dyspnea score, CAT= COPD Assessment Test, ICS= Inhaled Corticosteroids, GOLD= Global initiative for Chronic Obstructive Lung Disease, BMI= Body Mass Index, FFMI= Fat Free Mass Index, 6MWD= 6 Minute Walking Test Distance, Hypovitaminosis D= 25 OH vitamin D < 30 ng/mL, iPTH= intact Parathyroid Hormone, CRP= C-reactive protein, 25O(H)D= 25OH Vitamin D.
Serum SOST levels showed a positive correlation with age (r = 0.361, p < 0.001), BMI (r = 0.187, p = 0.03), FFMI (r = 0.382, p < 0.001), 6MWD (r = 0.192, p = 0.024), FVC (%) (r = 0.223, p = 0.011), FEV1 (%) (r = 0.265, p = 0.002) and DLCO (%) (r = 0.385 p < 0.001), and a negative correlation with CAT (r = -0.235, p = 0.005). Otherwise, SOST did not correlate with C-reactive protein. In multivariate analysis, only age (beta=0.264; p = 0.009), and FFMI (beta=1.241; p = 0.005) remained significant, although we also found a weak, positive, trend towards significance with DLCO (beta=0.074; p = 0.084).
Serum SOST levels showed a significant negative correlation with serum phosphorus (r = -0.291, p < 0.001) and β-Crosslaps (r = -0.167, p = 0.05), and a nearly significant correlation with P1NP (r = -0.165, p = 0.052). Serum SOST levels did not correlate with 25(OH) vitamin D, iPTH, total calcium, ionic calcium, alkaline phosphatase, albumin or magnesium levels. Finally, serum β-Crosslaps correlated positively with P1NP (r = 0.575, p < 0.001).
Predictors of SOST levelsUnivariate logistic regression indicated that low levels of SOST were associated with age (p < 0.001), sex (p = 0.002), DLCO (p = 0.042), FFMI (p < 0.001) and 6MWD (p = 0.014) at baseline, but not with smoking status, the Charlson index, mMRC dyspnoea score, FEV1 or the frequent exacerbator phenotype.
In addition, multivariate logistic regression (Table 2) revealed that low levels of SOST were independently associated with FFMI (OR 1.936 (IC95% 1.238–3.028) p = 0.004) but not with the other variables.
Associations between chronic obstructive pulmonary disease characteristics and low sclerostin levels (sclerostin ≤ 20 pmol/mL) using multivariate logistic regression.
FEV1= Forced expiratory Volume in the first second, mMRC= modified Medical Research Council Dyspnea score, High risk of exacerbation= 2 or more moderate exacerbations during previous year. DLCO= Diffusing Capacity of Carbon Monoxide, FFMI= Fat Free Mass Index, 6MWD= 6 Minute Walking Test Distance, Low sclerostin = sclerostin ≤ 20 pmol/mL.
Bold font indicates statistical significance.
A total of 139 patients were followed up for 12 months: 55 patients were in the low SOST group (SOST levels in the lower tertile <20 pmol/l) and 84 patients were in the high SOST group (SOST levels higher than the lower tertile). Table 1 shows the clinical characteristics of both groups.
During the 12-month follow-up period, 57 patients presented moderate COPD exacerbations (31 in the low SOST group), and 15 patients were hospitalized (11 in the low SOST group).
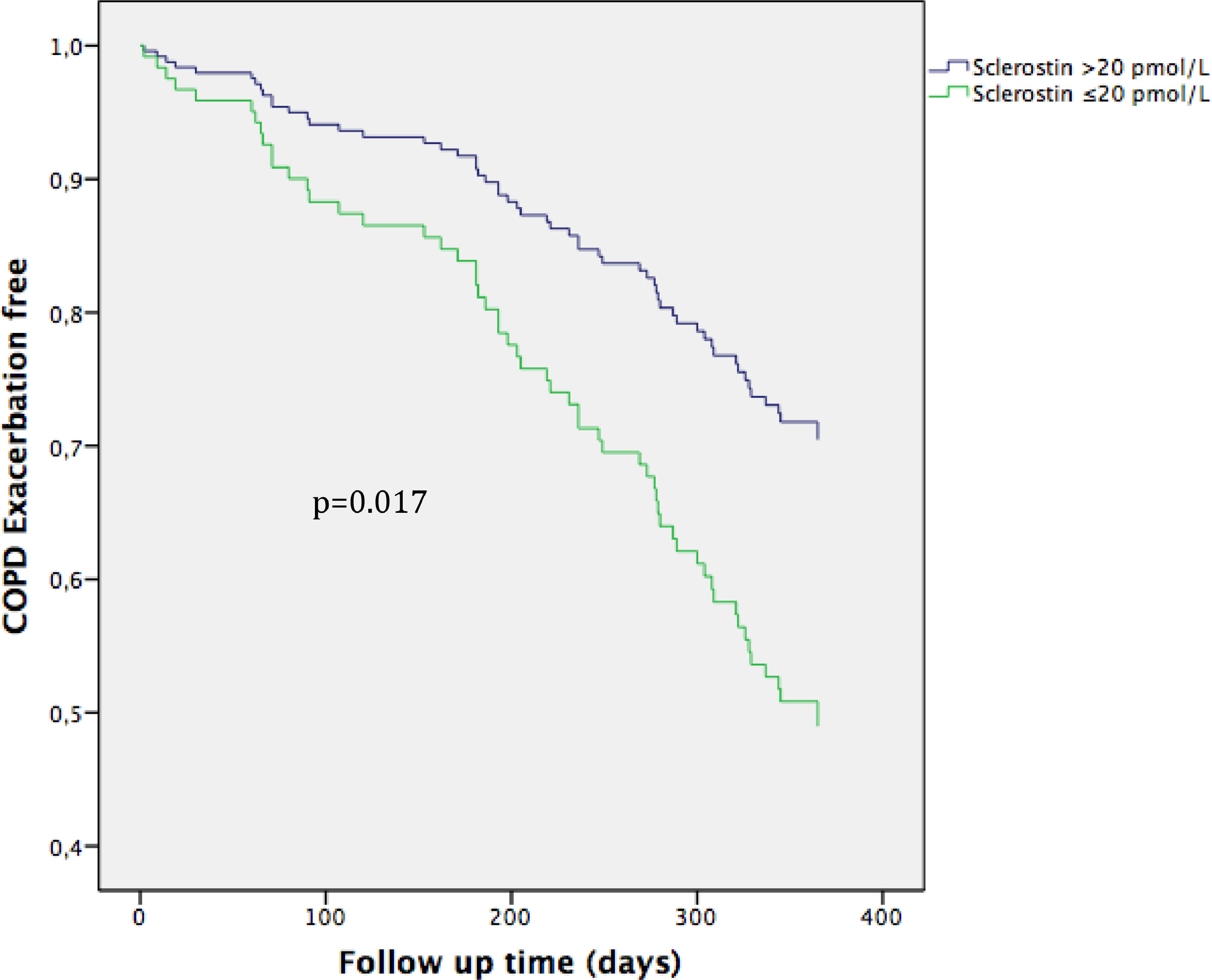
Univariate Cox proportional risk analysis indicated that low SOST (p = 0.001), FEV1 (p = 0.011), previous moderate COPD exacerbations (p = 0.002) and male sex (p = 0.007) were risk factors for COPD exacerbations, whereas age, BMI, use of inhaled glucocorticosteroids, Charlson index or smoking status were not. Multivariate Cox proportional risk analysis revealed that low SOST (HR 2.015, CI95% 1.136–3.577, p = 0.017) was a risk factor for moderate COPD exacerbation (Fig. 4; Table 3).
The curve representation for COPD exacerbation events as “cumulative survival from COPD exacerbation events” (on the y axis) during 365 days of follow-up (on x axis) comparing low Sclerostin patients versus the rest of the patients. Cox regression adjusted for age, sex, smoking status, Charlson index, modified Medical Research Council dyspnea score, FEV1 (predicted %), and high risk of exacerbation.
Cox regression analysis of predictors of COPD exacerbation.
FEV1= Forced Expiratory Volume in the first second, mMRC= modified Medical Research Council Dyspnea score, High risk of exacerbation= 2 or more exacerbations during previous year, Low sclerostin = sclerostin ≤ 20 pmol/L.
Bold font indicates statistical significance.
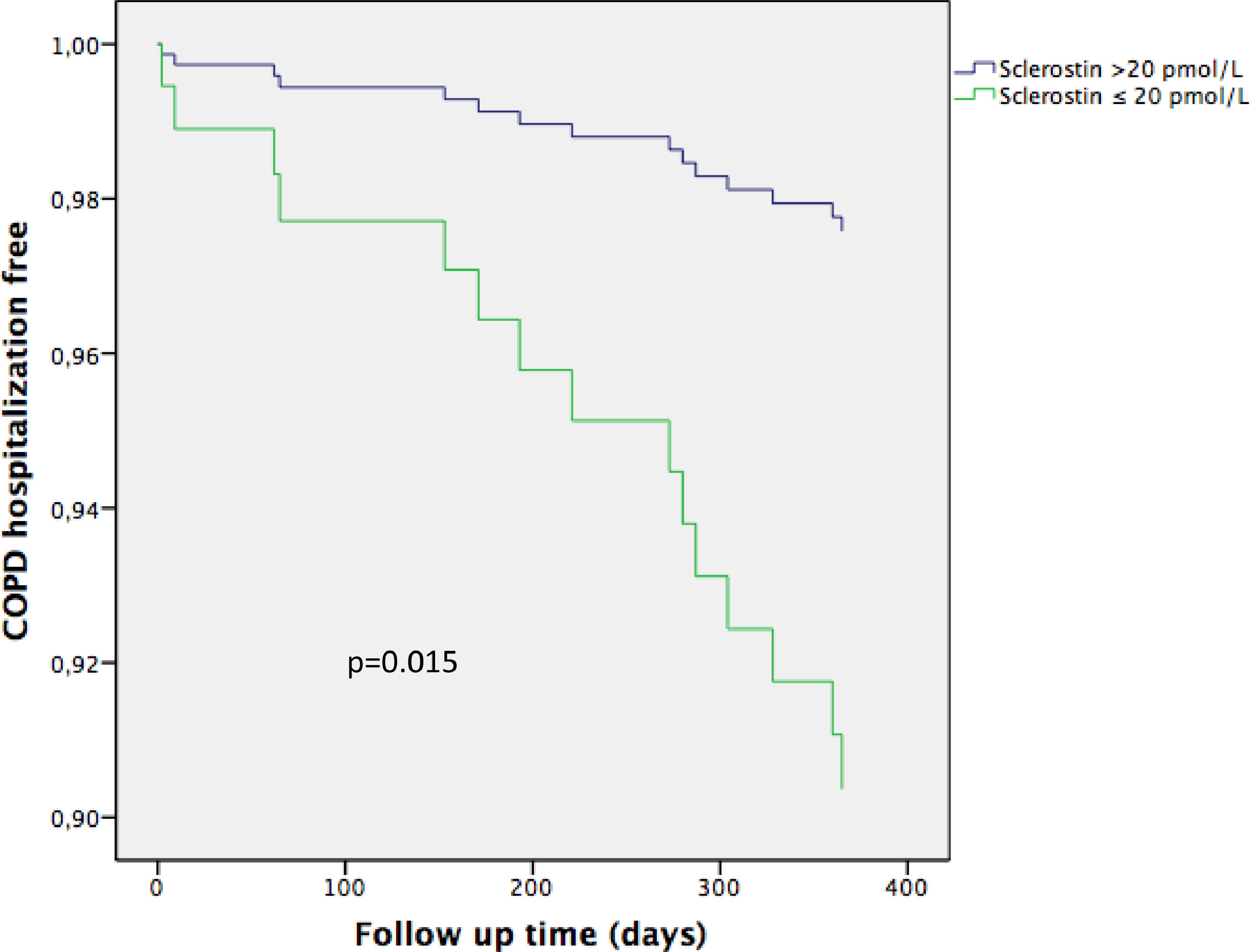
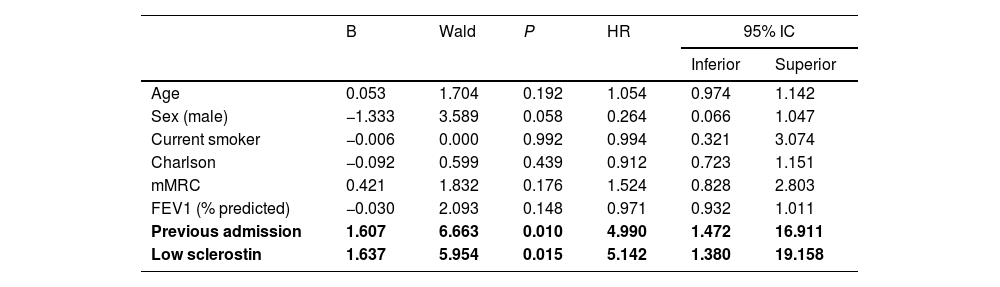
Univariate Cox proportional risk analysis showed that low SOST (p = 0.005), FEV1 (p = 0.001), previous admission due to COPD exacerbation (p = 0.001) and mMRC dyspnoea score (p = 0.001) were risk factors for hospitalization, whereas age, sex, use of inhaled glucocorticosteroids, the Charlson index and smoking status were not. Multivariate Cox proportional risk analysis indicated that previous admission (HR 4.99, CI95% 1.472–16.911, p = 0.01) and low SOST (HR 5.142, CI95% 1.380–19.158, p = 0.015) were risk factors for hospital admission (Fig. 5; Table 4).
The curve representation for COPD hospitalization events as “cumulative survival from COPD exacerbation events” (on the y axis) during 365 days of follow-up (on x axis) comparing low Sclerostin patients versus the rest of the patients. Cox regression adjusted for age, sex, smoking status, Charlson index, modified Medical Research Council dyspnea score, FEV1 (predicted %), and previous admission.
Cox regression analysis of predictors of hospitalization due to COPD exacerbation.
FEV1= Forced expiratory Volume in the first second, mMRC= modified Medical Research Council Dyspnea score, Low sclerostin = sclerostin ≤ 20 pmol/mL.
Bold font indicates statistical significance.
Our study showed that SOST correlates with several parameters in COPD, including body composition and lung function. Low levels of SOST are associated with lower muscle mass. Our results also showed a trend with the DLCO, thus suggesting that this biomarker may be associated with the emphysema phenotype. Importantly, low SOST levels predicted exacerbations and hospitalizations in COPD.
We studied a representative group of COPD outpatients from our clinic and excluded the most important exogenous factors known to modify SOST levels, such as kidney failure, calcium metabolism diseases or drugs known to interfere with bone metabolism. However, our cohort had a high prevalence of hypovitaminosis D and secondary hyperparathyroidism. The SOST levels were slightly higher in men, and a significant positive correlation with age was observed, as has been shown in the normal population19 and in other diseases.14,20 SOST levels also weakly correlated with BMI, a classical but limited marker of fat mass that has controversially been associated with SOST in the normal population.14,21,22 An interesting finding in our study was the significant positive correlation of SOST levels with FFMI, an important prognostic factor in COPD23 with an intrinsic association with lean mass. This finding, together with the association of SOST with DLCO, suggests that SOST is likely to be associated with a particular COPD phenotype. Although the definition of COPD phenotypes varies among studies,24 SOST has been associated with the multi-organ loss of tissue (MOLT) COPD phenotype25 and the pulmonary cachexia phenotype.24
We can only speculate about the possible causes and effects of low SOST in COPD. SOST is associated with muscle mass and exercise performance. SOST is accepted to be a myokine -a molecule produced by muscle and secreted during exercise.26 We found that SOST and FFMI were positively associated in patients with COPD; therefore, lower levels of circulating SOST might reflect sarcopenia. Other factors potentially involved in low SOST levels are inhaled glucocorticoid therapy and the association of low levels of vitamin D and high levels of PTH,7–8 although we did not find this association in our cohort. Regarding the effects of low levels of circulating SOST, one possible deleterious consequence in both the bronchi and lung parenchyma is that the Wnt pathway effect is not sufficiently downregulated, thus further diminishing lung function. This finding may explain the positive correlations with FVC, FEV1, DLCO and SOST.
Regarding bone metabolism, we observed a significant negative correlation of SOST levels with β-Crosslaps and a trend with P1NP. These results are consistent with the physiological function of SOST. Furthermore the β-Crosslaps and P1NP levels correlated positively, thus suggesting the existence of coupling between bone destruction (β-Crosslaps) and formation (P1NP). Similar degrees of correlation between SOST and bone remodelling markers have been found in other situations.27
Finally, we found that the group of patients with SOST in the lowest tertile had greater risk of exacerbation and hospitalization. This finding might be explained by the lack of inhibition of the Wnt pathway in patients with lower levels of SOST, thus favouring the dysfunction of bronchial epithelium and immunity.11 Furthermore, low levels of muscle mass are associated with exacerbations.4
Our study has several limitations. First, this was a single centre study; therefore, these results should be replicated in larger multicentre studies in patients with normal levels of 25(OH)D and PTH, because our population had a high prevalence of hypovitaminosis D. Moreover, our study reveals only associations but not causality. Specifically designed studies are necessary to demonstrate causality. Nonetheless, this question has potentially important clinical repercussions, because the antiosteoporotic agent romosozumab (an antibody blocking SOST) may potentially induce COPD deterioration, whereas a SOST agonist could inhibit COPD deterioration.
The main strengths of our study are its specific design for evaluating the possible effect of SOST on COPD exacerbations in a group of well-selected, well-characterized patients without diseases or drugs that could have altered the results, and the wide evaluation of bone metabolism as well as important COPD characteristics.
ConclusionIn conclusion, our study provides the first evidence that SOST is associated with several clinical outcomes in COPD, may be associated with emphysema phenotype, and may have a role as a biomarker to evaluate the risk of exacerbation and hospitalization in COPD. Further studies in other populations are needed to evaluate the potential therapeutic aspects that may be derived from these findings.
Author contributionsConceptualization: C.A.A., M.G.U. Data curation: P.F., S.T.M., C.A.A., C.V., A.B. Formal analysis: P.M. Project administration: C.A.A., P.M. Methodology: C.A.A., M.G.U., B.A.L., A.R.G., C.C., S.T., A.B. Resources: C.A.A., J.A., S.T., P.F., P.M., C.V. Visualization: C.A.A. Supervision: M.G.U., P.M. Software: P.M. Writing original draft: C.A.A., C.C. Writing review and editing: C.A.A., S.T., P.F., J.A., M.G.U., B.A.L., ARG, PM, CC.
Statement of EthicsThis study complies with internationally accepted standards for research practice and reporting. The study was approved by the Ethics Committee of our institution (2017.035). All patients provided written informed consent to participate in this study.
This study was funded by the Instituto de Investigación Sanitaria of Cantabria (IDIVAL).