In order to improve the quality of chronic obstructive pulmonary disease (COPD) patients' care, better knowledge of clinical practice and the factors associated with patient outcomes are needed. This study aimed to evaluate the relation between clinical practice and the outcomes of patients admitted for COPD exacerbations in Portuguese hospitals.
Materials and MethodsObservational, multicentre, prospective study with a 60-days follow-up period, in 11 hospitals, including patients aged ≥ 30 years, admitted to hospital for at least 24 hours due to an acute exacerbation of COPD. Demographic and clinical data were collected, including sex, age, smoking habits, hospitalisations, pulmonary function, comorbidities, COPD symptoms, and treatment. Sixty days after discharge, COPD exacerbations management, outcome measures, and readmission data were evaluated through a structured phone follow-up interview.
Results196 patients were included (85.7% male, mean age 71.2 years), the majority admitted through the emergency service. Ex-smokers and current smokers accounted for 51% and 36%, respectively. On admission, 72.4% were on LAMA, 54.6% on LABA, and 45.5% were on LABA/LAMA. Inhaled corticosteroids (ICS) were used in 37.3% and systemic steroids (SCS) in 10.3%. 35.7 % had had at least one exacerbation, with hospitalisation, in the previous year. There was no spirometry data for 23.2%. On hospitalisation, 98.5% of patients were treated with oxygen and 38.3% with non-invasive ventilation. Additionally, 93.4% used SCS and 60.2% ICS. Antibiotics were administered to 85.2%. 95.4% of patients were discharged; 9 died, 5 of whom had a COPD-related death. The median length of stay was 12 days for discharged patients and 33 days for patients who died. At discharge, 79.1% were prescribed with LAMA, 63.6% SCS, 61.5% LABA and 55.6% LAMA+LABA. 26,2% were prescribed with ICS+LABA+LAMA. At follow-up, 44.4% had a scheduled medical appointment within the 60 days after being discharged, and 28.3% were later readmitted due to exacerbation, of whom 52.8% were hospitalised.
ConclusionsThe severity of COPD, particularly in exacerbations, is directly related to impaired lung function and quality of life, mortality, and significant health system costs. Knowledge about COPD exacerbations' management in acute hospital admissions in Portugal may help stimulate a national discussion and review of existing data to engage clinicians, policymakers, managers, and patients, raising awareness and promoting action on COPD.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbimortality worldwide, imposing substantial individual, social and economic burdens. Its impact is expected to rise in the upcoming decades due to the continued exposure to risk factors and an accelerated ageing population.1–7 Although epidemiological data are irregularly distributed worldwide, the estimated worldwide COPD mean prevalence is 13.1% (95% CI 10.2–15.6%), and 12.4% in Europe (95% CI 8.8–16.0%).8 With the change of smoking habits in recent decades, and although still higher in men, the prevalence and mortality of COPD have increased more rapidly in women.9
Due to its high prevalence and chronicity, COPD causes substantial resource utilisation with frequent visits to clinicians, frequent hospitalisations due to acute exacerbations, and the need for chronic therapy, creating significant challenges for healthcare systems.2,5,10
Available therapy can improve symptoms and quality of life and prevent acute worsening of the disease.4 Likewise, COPD-appropriate management can decrease symptoms (especially dyspnea), reduce the frequency and severity of exacerbations, hospitalisations and readmissions, improve health status and exercise capacity, and prolong patient survival.5,11–15 Recently, there has been great interest in the study of COPD phenotypes and with the personalization of the treatment of COPD based on a phenotypical approach, there has been a change in non-pharmacological and pharmacological management of COPD.16
A better knowledge of the clinical performance and the identification of different factors associated with patients' outcomes are crucial to improving the quality of care for COPD patients17.
Furthermore, understanding all levels of standard practice can provide an opportunity for targeted quality improvement interventions that could significantly impact patients' care.18
Several observational studies, have shown COPD management in real-life setting and provide useful information for establishing appropriate clinical care. Esteban and colleagues identified that dyspnoea at baseline was the main variable associated with 30-day mortality after an emergency department evaluation for exacerbation of COPD, followed by the presence of cardiac disease, age, use of inspiratory accessory muscle/ paradoxical breathing, and the Glasgow Coma Scale score.19 Another study found that the factors associated with a higher probability of hospital admission of COPD patients due to respiratory causes were smoking and receiving oxygen therapy, whereas re-admissions were associated with being men, aged 70 years and over, and a smoker.20 These factors, as shown by Terzano and colleagues have implications to the length and cost of hospitalization.21
This study aimed to evaluate the clinical practice, clinical factors, and the outcomes of patients admitted for COPD exacerbations in Portuguese hospitals. Secondary objectives included characterisation of disease profile in hospitalised patients, the assessment of treatment and resources used; patients' outcomes assessment at 60-days after hospital discharge; and compliance with the GOLD guidelines' appraisal.
Material and methodsStudy designObservational, multicentre, prospective study with a 60-days follow-up period.
PopulationMale and female patients aged ≥ 30 years, admitted to hospital for at least 24 hours due to an acute exacerbation of COPD, who have given their written informed consent for study participation.
Data collection and study assessmentsDuring the screening phase of 16-weeks, all consecutive cases admitted to hospital with a suspicion of a COPD exacerbation were identified upon admission, and the patients were invited to participate in the study. At discharge, if COPD exacerbation was confirmed as the cause of admission and the eligibility criteria were met, the patient was included in the study. The data were retrospectively recorded).
For eligible patients, demographic and clinical data were collected, including sex, age, patients' referral, smoking habits, hospital readmissions, pulmonary function, comorbidities, and specific data on COPD as COPD symptoms and treatment. At follow-up, 60 ± 7 days after discharge, COPD exacerbations management, outcome measures, and readmission data were evaluated through a structured phone interview. Outcomes included in-hospital mortality, length of hospitalisation, hospital readmissions, and mortality at 60-days after hospital discharge.
Ethical aspectsThis study was approved by the study sites' institutional review boards or ethics committees.
The work described has been carried out according to the International Conference on Harmonization of Good Clinical and Pharmacoepidemiological Practices, in agreement with the Declaration of Helsinki and maintaining patients' confidentiality.
Written informed consent was obtained from all individual participants involved in the study.
Statistical analysisDescriptive statistics were calculated for all variables. Mean, median and interquartile range were used for quantitative variables and the absolute and relative frequencies for qualitative ones. Statistical analysis was performed with R software.
ResultsSites and recruitmentEleven hospitals participated in the study, and between December 2016 and July 2017, 196 patients were included (Table 1). The follow-up period was complete on 30th September 2017.
Participant hospitals and recruitment.
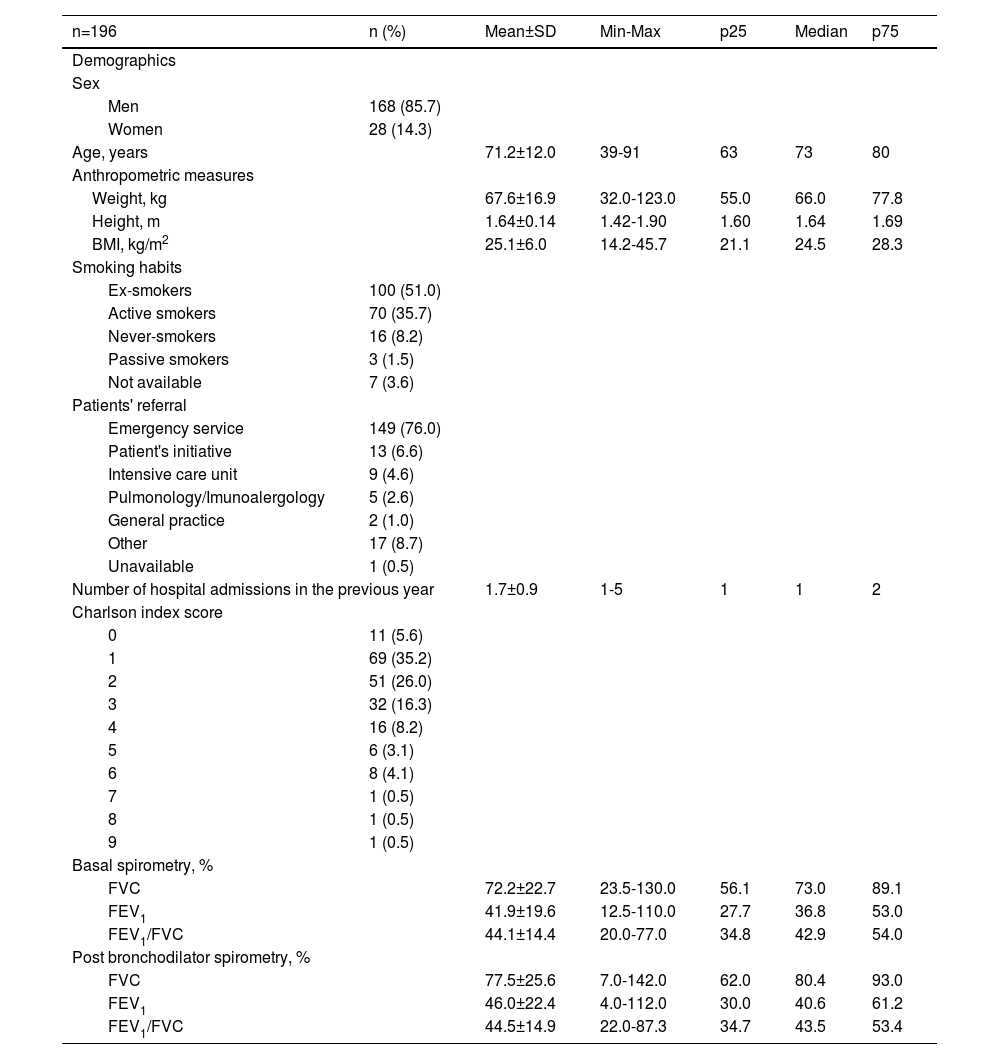
Most patients were male (85.7%), mean age was 71.2 years old and most were admitted through the emergency service (76.0%). Ex-smokers and active smokers represented 51.0% and 35.7% of the sample, respectively, and 35.7% had been hospitalized at least once for COPD exacerbation in the preceding year. Charlson's comorbidities index score was between 1 and 3 for 77.5% and ≥4 for 16.8% of the enrolled patients. Regarding respiratory symptoms, 54.1% of the admitted patients presented worsening dyspnea, increased sputum production, and change in sputum colour; 24.5% with only one symptom (96.9% with worsening dyspnea, 71.9% had increased sputum production, and 55.6% a change in sputum colour). The demographic and clinical characteristics of the patients included in the study are summarised in Table 2.
Patients' demographic and clinical data at baseline.
BMI – Body Mass Index, FEV1 - forced expiratory volume in 1 second, FVC - forced vital capacity, Max – maximum, Min – minimum, n – number of patients, p25 – 25th percentile, p75 – 75th percentile, SD - standard deviation.
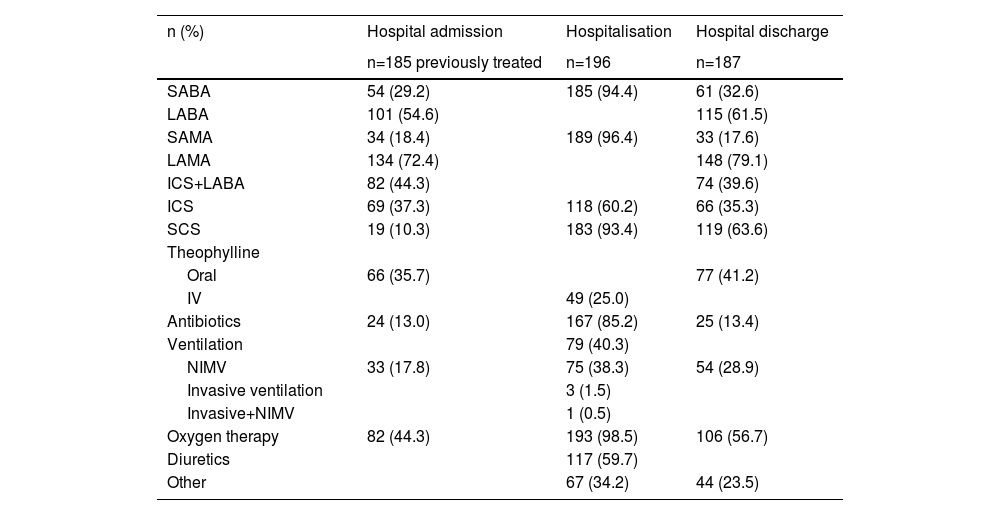
On admission, most patients (94.4%) reported previous medication. Of these, 72.4% were treated with long-acting anticholinergic receptor antagonists (LAMA) and 54.6% with long-acting β2-adrenoceptor agonists (LABA). Of the 185 patients who reported previous medication, 53.0% were under ICS + LABA + LAMA, 17.5% were treated with LABA + LAMA, and 10.3% used systemic corticosteroids (SCS) (Table 3). Although patients met the study inclusion criteria, due to informatics difficulties in some hospitals, spirometry data records were missing for 23.2% of the patients.
Treatment at hospital admission (last 30 days), during hospitalisation, and at hospital discharge.
ICS - inhaled corticosteroids, IV – intravenous, LABA - long-acting β2-adrenoceptor, LAMA - long-acting muscarinic receptor antagonists, NIMV - noninvasive mechanical ventilation, SABA - short-acting beta-agonist, SAMA - short-acting anticholinergic, SCS - systemic corticosteroids.
Most patients were on oxygen therapy (98.5%), and 38.3% were treated with non-invasive ventilation. Additionally, 93.4% used SCS and 60.2% ICS. Antibiotics were also a regular part of treatment and were administered to 85.2% of the patients (Table 3).
Nine patients died during hospitalization, five of whom had a COPD-related death. The median length-of-hospitalization (±SD) was 12 ± 10 days for discharged patients (95.4%) and 33 ± 16 days for patients who died.
Hospital dischargeOxygen therapy was prescribed to 56.7% of discharged patients. At discharge, 79.1% of the patients were prescribed LAMA, 63.6% SCS and 61.5% LABA; 55.6% LAMA+LABA, 51.0% of which were also prescribed an ICS; and 26.2% were prescribed ICS+LABA+ LAMA (Table 3). Spirometry results were available for 5.9% of the patients.
A medical appointment and exams were scheduled within 60 days after discharge for 94.1% (n=176) and 27.3% (n=51) of the discharged patients.
60 days follow-upFollow-up was completed for 96.3% (n=180) of the discharged patients. Three patients died after hospital discharge; two of these deaths were COPD-related.
Of the 187 discharged patients, 44.4% (n=83) had the medical appointment scheduled at discharge, and 28.3% (n=53) were admitted to the emergency department due to a COPD exacerbation. From these, 52.8% (n=28) were hospitalised. Spirometry results were available for 9.5% of the patients, 75% of whom had a scheduled medical appointment (Table 4).
Spirometric data at hospital discharge.
| % | Mean±SD | Min-Max | p25 | Median | p75 |
|---|---|---|---|---|---|
| Hospital discharge, n=187 | |||||
| FVC | 74.6±25.3 | 59.0-100.0 | 63.5 | 70.0 | 83.5 |
| FEV1 | 49.5±21.1 | 21.0-72.0 | 43.5 | 53.0 | 59.0 |
| FEV1/FVC | 48.0±19.1 | 27.0-70.0 | 40.0 | 48.0 | 55.0 |
FEV1 - forced expiratory volume in 1 second, FVC - forced vital capacity, Max – maximum, Min – minimum, n – number of patients, p25 – 25th percentile, p75 – 75th percentile, SD - standard deviation.
COPD is a leading cause of death worldwide, representing a substantial and increasing socioeconomic burden.6 Since COPD is associated with long-term exposure to toxic gases and particles, more people will express the long-term effects of exposure to risk factors as longevity increases.1,2,6 COPD prevalence is expected to rise over the next 40 years: by 2060 over 5.4 million deaths per year from COPD and related conditions are estimated.6,22
Disease clinical course can vary from years of stability to devastating acute exacerbations and respiratory failure, symptoms ranging from chronic productive cough to debilitating dyspnea.23 An increasingly severe inflammation characterises disease worsening during stable condition and by an intensifying frequency of exacerbations, the most relevant event affecting COPD mortality.6,11,24
The severity of COPD is directly related to the cost of care, and the cost increases as the disease progress. COPD exacerbations are a major cause of the high 30-day hospital readmission rates associated with COPD.14 Severe COPD exacerbations that result in hospitalisation can be up to 60 times more expensive than mild or moderate exacerbations managed by primary care services.25 Moreover, exacerbations are associated with impaired lung function and quality of life, mortality, and significant health system costs, accounting for the greatest proportion of the total COPD burden on healthcare systems.6,7,14,18,26 Morbidity measures traditionally include physician visits, emergency department visits, and hospitalizations. Available data indicate that COPD morbidity increases with age and may be affected by concurrent chronic conditions that are smoking-, ageing- and COPD-related, which may interfere with disease management and are the primary drivers of hospitalisations, and costs for patients with COPD.6,27–30
Patients with COPD have a mortality rate higher than the general population, depending on their disease severity.31,32 However, the reduced accuracy of mortality data is associated with the under-recognition and under-diagnosis of COPD, which, together with the uncertainty of diagnosis, contribute to underestimating the disease and its burden.2,6,26,33 The high prevalence of COPD coupled with the widespread under-recognition and under-diagnosis of the disease stresses the need to raise COPD awareness.34,35
Aiming to know the Portuguese reality regarding COPD, COPD exacerbations and its management, and to compare it with the GOLD guidelines, this study analysed the participating hospitals' clinical outcomes and organisational data, providing evidence to support a better organization of patients’ care and improved use of resources within Portugal.
Consistently with the literature, the patients included in the study were mostly older men (mean age 71 years old, male to female ratio 6:1), a third of whom still smoked, with moderate to severe spirometric disease, a history of hospitalisation for COPD exacerbations and several comorbidities.
Readmissions for recurrence of exacerbations in this population were also frequent, as found by the European COPD audit involving > 400 hospitals that reported a 90-day readmission rate of 35.1%.36 Similar results were found in the ECLIPSE study, in which COPD exacerbations requiring hospital admission were relatively frequent events occurring in about 30% of patients during the 3-year follow-up.37 In Portugal, frequent acute exacerbations were reported by 38.0% of stable COPD outpatients over 40 years old.38 The mortality was lower than in previous studies involving these patients (exacerbators with poor lung function).37 This difference might be explained by the more common use of non-invasive ventilation in Portuguese hospitals, shown to reduce mortality in COPD patients.39,40 Nasal high flow oxygen therapy has been used for the management of respiratory failure, complementarily and as an alternative non-invasive respiratory support.41 However, little and limited evidence for improved clinical outcomes is currently available.
Telemonitoring can be important to predict exacerbations by monitoring symptoms and biological variables such as oximetry, physical activity and temperature. It may also be useful in post-exacerbation discharge and to avoid re-aggravation of symptoms and re-exacerbations.42
Compliance with GOLD guidelines regarding diagnosis was established, as COPD diagnosis was considered for patients with dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease, and a post-bronchodilator FEC1/FVC < 0.70 confirming the presence of persistent airflow limitation. To define therapy, COPD assessment must consider the presence and severity of spirometric abnormalities, current nature and magnitude of patients' symptoms, exacerbation history, and risk of future events, namely exacerbations, hospital admissions, death, and presence of comorbidities.12
Pharmacologic therapy for COPD is used to treat symptoms, reduce the frequency and severity of exacerbations, and improve exercise tolerance and health status. The factors associated with successful treatment should be considered, not only those relying on patients, but also on health-care providers and on the relation between them. One study in Portuguese COPD patients showed that the adherence to medication was higher in patients with more symptoms and more obstructed, whereas previous COPD exacerbations improved prescribers’ adherence to treatment guidelines.43 However, it failed to prove a positive association between non-adherence to medication, inhalers mishandling or prescribers’ non-adherence to GOLD strategy with symptoms, exacerbations, and airflow limitation.43
In this study, the medications used to treat COPD included bronchodilators LABAs and/or LAMAs and ICS, and recommended treatments ranged from a single short-or long-acting bronchodilator to combinations of two bronchodilators and ICS. This is compliant with the international guidelines regarding in-hospital treatment and shows a high standard of care.6,12,26,44
The small sample size is the main limitation of the study. Although the incidence of COPD is high, the Portuguese centres still have some difficulties to overcome in the development of clinical investigation studies, including the low organisational capacity which causes some barriers to the recruitment of patients. Additionally, in some hospitals, these organisational problems include difficulties with informatics, and there was a lack of information, namely spirometric data, not because they did not exist, but because they were unavailable at the moment of data registry for the study. The development of studies with these characteristics may help to professionalise clinical investigation and, ultimately, with more knowledge about the global panorama, to improve patients’ care.
ConclusionsThe presented COPD study evaluated COPD exacerbations' management in acute hospital admissions in Portugal, aiming to raise the standards of care to a level consistent with the GOLD management guidelines. Furthermore, the results obtained from this study may help stimulate a national discussion and review of existing data to engage clinicians, policymakers, managers, and patients, raising awareness and promoting action on COPD.