Bronchial mucous gland adenoma (BMGA) is a very uncommon, benign entity, defined as a proliferation of seromucous glands in the bronchial lamina propria, without cellular atypia,1 usually arising in the proximal airways,2 with some rare reports of peripheral3 and endobronchial localization.4 BMGA does not have gender predilection, with mean age of 52 years,5 but rarely can present in children.6
A 50 years-old male, former smoker (30 packages/year) had chronic cough complaints with 2 years evolution, not under any relevant medication.
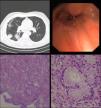
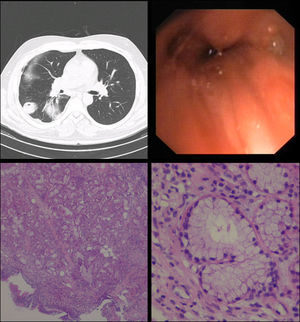
Cardiac auscultation revealed rhythmic beatings without murmurs and on pulmonary auscultation lung sounds were normal. Respiratory function tests were also normal (vital capacity – 99%; FEV1 – 80%). Radiology (X-ray) was unspecific. Thorax computed tomography (CT) scan was requested – right lower lobe atelectasis (Fig. 1A). Fiberoptic bronchoscopy (FOB) was performed and granulation tissue was evident at the middle/lower bronchus division – biopsies only showed non-specific bronchial inflammation.
Due to continuation of clinical complaints and inflammatory FOB, a rigid bronchoscopy was executed at the right lower lobe bronchus and small protrusion was seen – biopsies were performed (Fig. 1B). At this point, microbiology studies isolated Klebsiella pneumoniae, ampicillin resistant in aspirate product.
Histological examination showed an exophytic lesion, of bronchial mucous glands, with monotonous basal nuclei and mucinous cytoplasm (Periodic acid–Schiff–PAS positive); immunohistochemistry showed positivity for cytokeratin 7 (SP52, Ventana, AZ-USA) and negativity for cytokeratin 5/6 (D5/16B4, Ventana, AZ-USA) and thyroid transcription factor (TTF1, SP141, Ventana, AZ-USA) (Fig. 1C and D). The final diagnosis of bronchial mucous gland adenoma was reported.
However, after a totally symptom-free period, the patient developed lower lobe pneumonia, motivating long antibiotic treatment with piperacillin/tazobactam, metronidazole and linezolid, which led to clinical improvement. One month later, CT scan was performed and revealed right lower lobe bronchiectasis and parenchymal densification, as well as organized pleural effusion.
Surgical intervention was considered and accepted. On surgery, the lower lobe was markedly atrophic and showed multiple adhesions to the thoracic pleural and diaphragm. Lobectomy was performed without complications.
The surgical specimen showed, on the main bronchus, a small fibrous scar with involvement of mucous glands, which was considered as corresponding to the stalk of the lesion first identified on the bronchoscopy and reported as bronchial mucous gland adenoma. The remaining lung revealed bronchiectasis with dense inflammatory infiltration, type 2 pneumocytes hyperplasia and macrophages in alveolar spaces.
The patient was discharged after surgery. The therapeutic plan was based on respiratory kinesiotherapy, prophylactic antibiotics and analgesic drugs. One month later patient was clinically well and without complaints.
BMGA is a rare and benevolent tumor of the tracheobronchial mucous glands, first described in 1882 by Muller,1 with few case reports in the literature – 10 cases representing the largest group.5 It has been reported in the literature under the name of adenomatous polyp, adenoma of mucous gland type, bronchial cystadenoma and papillary cystic adenoma.6
This tumor occurs equally in male and female gender, and at all ages.6,7 Clinical manifestations are the result of bronchial obstruction due to tumor growth and include haemoptysis, cough, dyspnea, wheezing, and sometimes pneumonia.4
Differential histological diagnoses include low-grade mucoepidermoid carcinoma, primary adenocarcinoma and other benign adenomatous lesions such as mucinous cystadenoma, papillary adenoma and alveolar adenoma.1 The absence of squamous and intermediate cells will exclude mucoepidermoid carcinoma7 and the lack of malignancy features such as cellular atypia, mitotic activity and invasive growth pattern should eliminate the diagnosis of adenocarcinoma.4 As for the other benign tumors, they usually are parenchymal lesions, with papillary growth pattern and variable proportion of cuboidal/goblet cells (papillary adenoma), cystic-like spaces lined by cubic cells (alveolar adenoma) and mucous lined cyst filled with mucin (mucinous cystadenoma); the first two are strongly positive for TTF-1 staining, with variable expression in the latter.1,2,4,7
BMGA is a rare tumor of the trancheobronchial tree, with unspecific signs and symptoms, which may delay the diagnosis for years. Recognizing this entity, both for pulmonologist and pathologist, is vital, in order to consider the hypothesis of diagnosis as well as the correct histological evaluation and appropriate therapy.
Conflicts of interestThe authors have no conflicts of interest to declare.