Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease caused by John Cunningham (JC) virus and occurs in immunosuppressed patients. PML affects mainly the cortical and subcortical white matter, includes neurological symptoms such as ataxia, hemiparesis, visual anomalies and can also be accompanied by behavioural alterations. PML can also cause lesions in other central nervous system (CNS) areas, such as the brainstem or the spinal cord.1,2
PML may occur in patients with severe brainstem injuries, such as tumours, strokes or infections which might affect the central chemoreceptor zone, causing central alveolar hypoventilation syndrome (CHS).5–7
We report the case of a 27-year-old woman who had undergone a double intestinal-kidney transplantation in 2013. Induction immunosuppression consisted of corticoids, tacrolimus and eculizumab. In 2014 she gradually developed diplopia, nystagmus, ataxia and dysphagia. A magnetic resonance imaging (MRI) showed a bilateral asymmetric hyperintense lesion in the left margin and the posterior part of the brainstem, the posterior part of the mesencephalon and on the three bilateral cerebellar peduncles. Patchy and punctuate gadolinium enhancement was identified over medulla. JC virus was demonstrated by polymerase chair reaction (PCR) in cerebrospinal fluid (CSF). Once the diagnosis of PML was confirmed, treatment with eculizumab was replaced with everolimus. Clinical improvement was noticed with the new treatment.
In 2015, the patient was hospitalized due to a three-day history of throbbing headache, sonophobia, photophobia, nausea and vomiting. Physical examination revealed a heart rate of 110 beats per minute, blood pressure of 114/65mmHg, temperature of 37°C and oxygen saturation using pulse oximetry (SpO2) of 87% even with oxygen therapy at 3 litres per minute. Diminished vesicular sounds in both lungs were heard during auscultation and the neurological examination was normal.
The results of the creatinine blood test were 2.38mg/dl (normal <1.2mg/dl), similar to previous levels, biochemistry and blood cell count were normal. Venous blood gas (VBG) results indicate a pH of 7.29, a pCO2 of 52mmHg (6.93kPa), pO2 48mmHg, (6.40kPa) and HCO3 of 25mmol/l. Findings in chest radiographs confirmed the presence of bilateral pleural effusion with right-side predominance.
During hospitalization, the patient experienced high fever and a decline in the level of consciousness. She was admitted to the intense care unit (ICU) and treated with non-invasive mechanical ventilation (NIV) support within the first 48hours. Diuretics and broad-spectrum antibiotics treatment were administered on the suspected respiratory infection associated with heart failure. Study of pleural fluid sample was obtained observing transudative effusion without any microbiological isolation. Blood, urine and sputum samples were not associated with microbiological aetiology.
When the patient's condition became stable in hospital, in the ward sleep apnea was observed. She complained of headaches on awakening, daytime sleepiness and poor nocturnal rest. Repeated arterial blood gas (ABG) revealed hypercapnia and mixed acidosis.
Respiratory polygraphy (RP) showed an apnea-hypopnea index (AHI) of 83/hour, 471 hypopneas and 49 central apneas. Oxygen desaturation index (ODI) was 135/hour and average time with SpO2 <90% was 91%. Average transcutaneous pCO2 level was 55.5mmHg (7.40kPa), with a maximum of 63mmHg (8.40kPa).
Nocturnal NIV was applied using an oronasal mask. The settings were a spontaneous/assisted mode, a pressure support of 10 and an expiratory pressure of 8cmH2O. After 48hours of treatment, clinical and gasometric improvements were observed.
The patient was discharged home with mechanical ventilation. Hyperventilation symptoms were checked. The efficacy of NIV was assessed with the built-in software and transcutaneous capnography. Normal values for spirometry test were obtained.
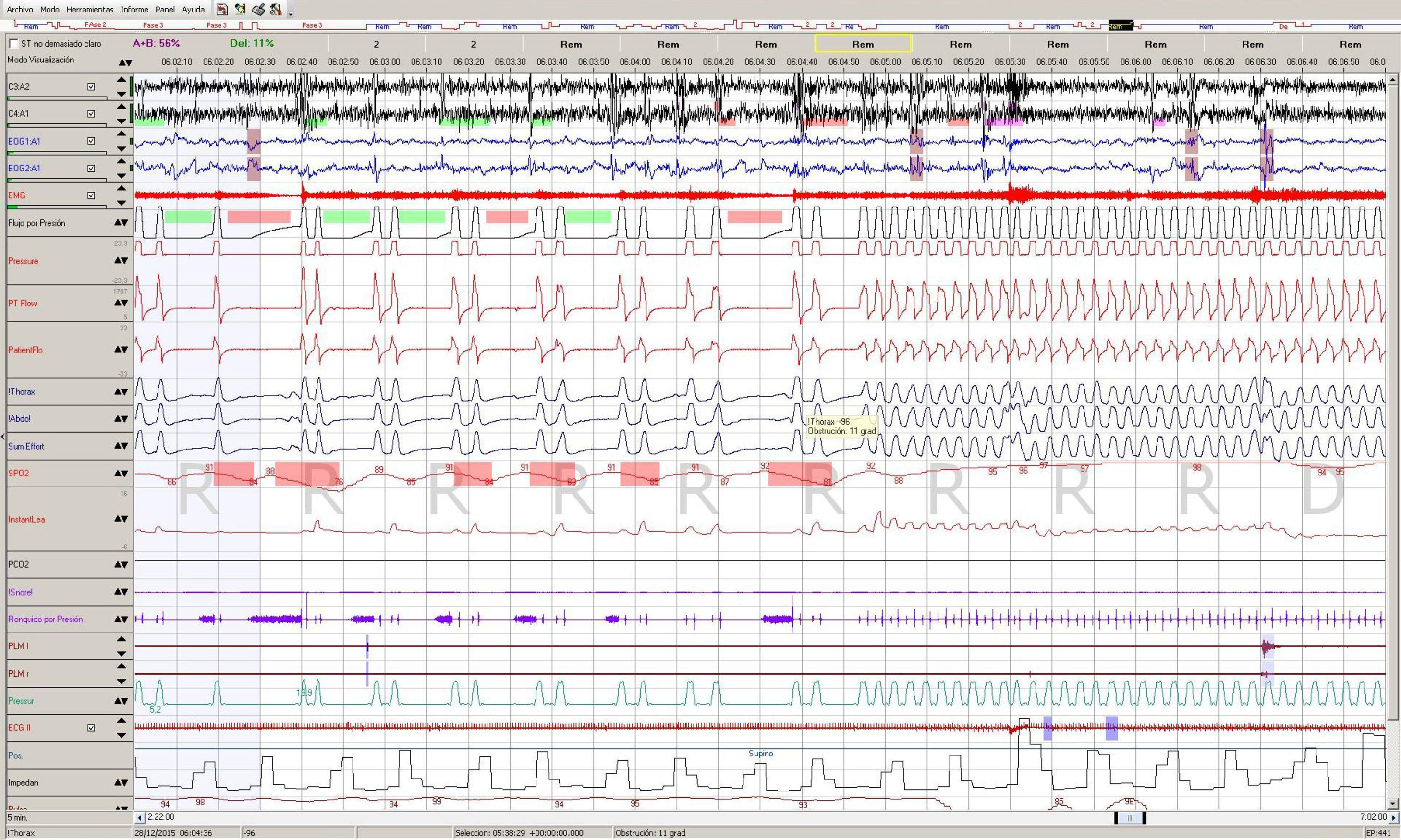
The evolution was favourable. Risk markers of PML were not found three months later (no new visible lesions on her MRI and the patient was with negative JC virus PCR), so the possible resolution of the central hypoventilation syndrome was evaluated. A baseline RP showed an AHI of 96/hour, 187 hypopneas and 344 apneas (89% central). The ODI was 99/hour and an ABG in the early morning showed a pCO2 of 46mmHg. We decided then to perform a polysomnography (PSG) with NIV to monitor the episodes of central apnea (Fig. 1). Those episodes were observed mainly during REM sleep only if we tried to change a spontaneous mode, and were resolved by switching to a spontaneous-assisted mode so we decided to maintain the NIV in that mode and to discharge the patient. The rest of the study was normal, with an IAH of 15.6 due to those mode changes. The patient was set on long-term home NIV, and hyperventilation symptoms resolved.
PML is a CNS disease that affects particularly immunosuppressed patients, e.g. HIV seropositive patients or following an organ transplant. It is produced by the JC virus which leads to a lytic infection of oligodendrocytes and astrocytes whose neurologic deficits correspond to areas of demyelination in the brain, frequently localized in the subcortical hemispheric white matter or the cerebellar peduncles. The disease can occur in other regions of the central nervous system including the brainstem, the optic nerves or the spinal cord. The PML presentation begins with focal neurologic deficits that depend on the location of the lesions. The diagnosis of the disease requires MRI, performing PCR for DNA JC virus or a brain biopsy.
Currently, there is no specific treatment for JC virus infection, although a few antiviral medications have been studied for treatment of PML. The only treatment available is the restoration of immune function through immunomodulatory therapies. In this case report, eculizumab was replaced by everolimus when PML was diagnosed. Viral load (measured by PCR in CSF) and immune response are significant factors for the prognosis, although more than half of the cases treated have some significant neurological consequences.1,2
According to The International Classification of Sleep Disorders (ICSD), alveolar hypoventilation secondary to medical condition is relatively uncommon.3 CHS can result from neoplasms, strokes, encephalitis and neurodegenerative conditions which can be developed as a result of severe injury or trauma to the brain or brainstem, but there are no reported cases of bulb lesions to PML disease.5–7
Our patient presented persistent brainstem injuries, symptomatic hypercapnia and demonstrated hypoventilation, showing the association between these alterations. Moreover, after management and outcome of negative JC virus PC, repeated episodes of central apnea by RP and PSG were observed. The medulla damage was considered irreversible, so long term NIV is prescribed.4,8,9
This case report reveals the importance of studies for the diagnoses of sleep disorders in patients with medulla infectious lesions. It shows how central apneas may cause central alveolar hypoventilation. Close monitoring of respiratory function and non-invasive ventilation support when it is needed are important in the treatment of these patients in order to improve survival and quality of life.
Conflicts of interestThe authors have no conflicts of interest to declare.