Chronic thrombo-embolic pulmonary hypertension (CTEPH) is the consequence of fibrotic organization of unresolved pulmonary emboli and secondary microvascular remodeling, that can ultimately lead to right heart overload, failure and death.1–4 It is associated with a high burden of disease with 5-year survival being reported as low as 10% for untreated patients.5 Depending on the nature and location of the disease, surgical pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA) and pulmonary vasodilator therapy (PVT) should be considered for proximal, distal and microcirculation lesions, respectively.1
The authors report a successful case of BPA and PVT combined treatment in a young patient with predominantly distal CTEPH. A 35-year-old Caucasian male, with hypertension, former smoking history and recurrent deep vein thrombosis, was referred to our pulmonary hypertension (PH) dedicated center because of low-effort fatigue (WHO class III) and signs of right heart failure. He had no history of syncope. At the time of admission, he was under long-term oxygen therapy (LTOT) (8 h/day) and warfarin (which he was only compliant with only 8 years after the first DVT episode).
Initial evaluation was compatible with severe right chambers pressure overload and a high probability of pH (Fig. 1). Impaired functional capacity was also documented, with a 6 min walking test distance of 270 m (37% of the predicted value), additional oxygen desaturation (92% to 88%) and blood pressure fall (BP 96/55 mmHg to 77/50 mmHg). Due to high probability of pH, the patient was submitted to a right heart catheterization (RHC) that confirmed pre-capillary PH with multiple high-risk criteria (Table 1). CTEPH was diagnosed after the etiologic workup revealed multiple and bilateral perfusion defects in ventilation/perfusion scan; blood tests (including liver and thyroid function, serologies and autoimmunity) and pulmonary function test were normal. Screening for acquired or genetic thrombophilia was also negative. Computed tomography pulmonary angiography was highly compatible with CTEPH and revealed multiple bilateral segmental and mostly subsegmental pulmonary artery filling defects (Fig. 1).
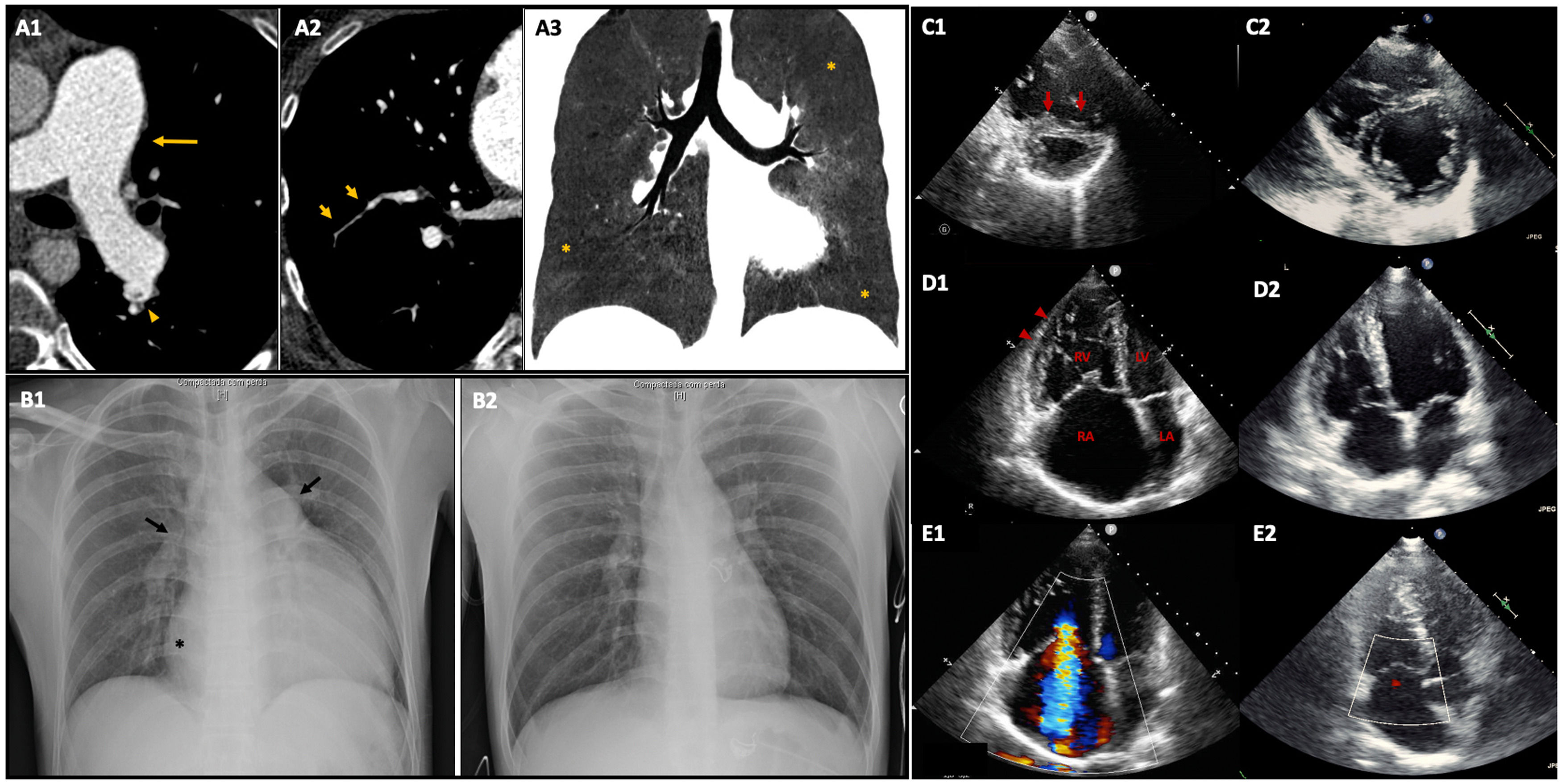
Imaging findings before and after combined treatment with pulmonary vasodilator therapy and balloon pulmonary angioplasty.
Capture A: Vascular and pulmonary findings of chronic thromboembolism on CT pulmonary angiography. (A1) and (A2) Axial plane images reveal main pulmonary artery dilation (arrow), linear filling defects within pulmonary arterial vessels – webs (arrowhead), caused by residual thrombotic material, and bronchial arteries dilation followed by abrupt transition of size, irregularity and stenosis of multiple peripheral arteries (short arrows). (A3) Mosaic perfusion pattern (MinIP reconstruction image, coronal plane) defined by variable lung attenuation and due to heterogeneity of lung parenchyma, in which hypoperfused peripheral regions have low attenuation (*) compared to those of normal lung perfusion.
Capture B: Comparison of chest X-ray before and after treatment (B1 and B2, respectively). (B1) Severe cardiomegaly, dilation of the right atrium (*), dilation of the main pulmonary artery and right pulmonary enlargement (arrows). (B2) Almost normal chest x-ray after treatment.
Captures C to E: Comparison of transthoracic echocardiogram images before and after treatment (C1-E1 and C2-E2, respectively). (C1) End-diastolic short-axis view showing severe right ventricle (RV) dilation and interventricular septum deviation to the left caused by RV pressure overload (interventricular septal D-shape – short arrows), significantly impairing left ventricle diastolic filling. (D1) and E1) Apical four-chamber end-diastolic view (D) and Doppler mid-systolic image (E) showing initial dilated and hypertrophied RV (arrowheads), severely dilated right atrium (RA), associated with severe tricuspid regurgitation with an estimated PSAP of 88 mmHg. After treatment there was a significant decrease in right chambers’ size with only mild tricuspid regurgitation and improvement of LV diastolic filling.
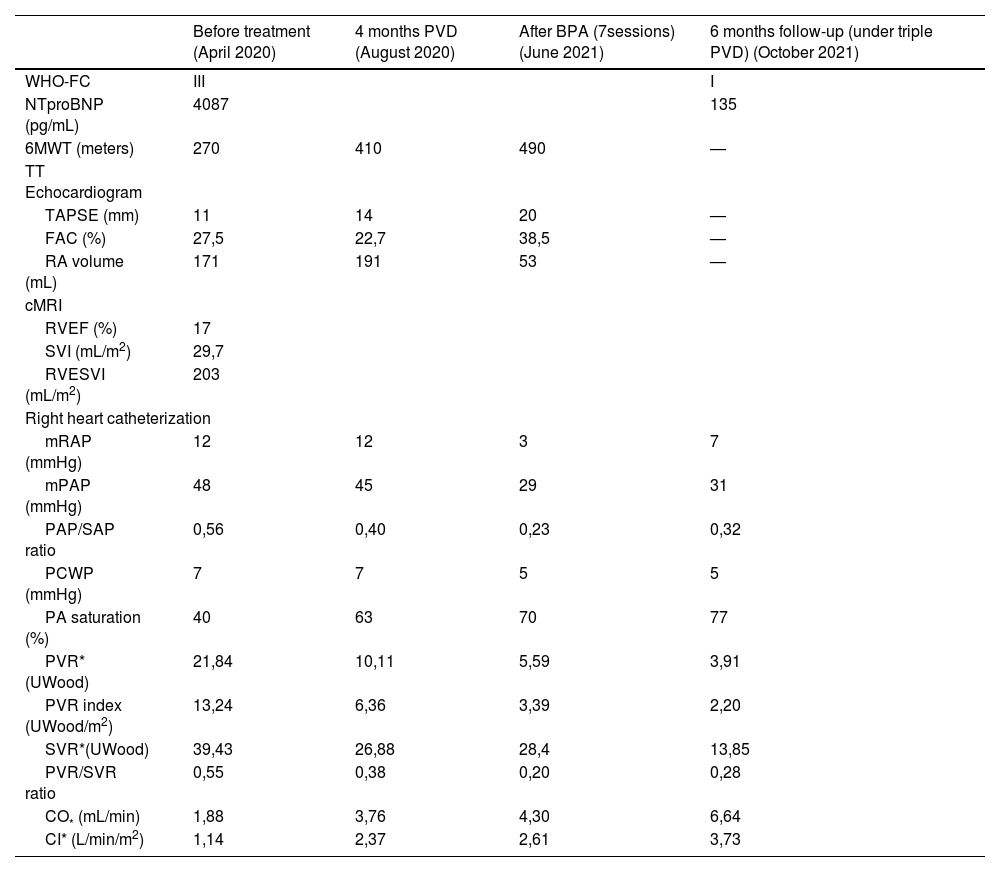
Clinical, imaging and haemodynamic parameters before treatment initiation, 4 months after pulmonary vasodilators introduction, after 7 sessions of BPA (under triple PVD) and 6 months of follow-up (under triple PVD).
| Before treatment (April 2020) | 4 months PVD (August 2020) | After BPA (7sessions) (June 2021) | 6 months follow-up (under triple PVD) (October 2021) | |
|---|---|---|---|---|
| WHO-FC | III | I | ||
| NTproBNP (pg/mL) | 4087 | 135 | ||
| 6MWT (meters) | 270 | 410 | 490 | — |
| TT Echocardiogram | ||||
| TAPSE (mm) | 11 | 14 | 20 | — |
| FAC (%) | 27,5 | 22,7 | 38,5 | — |
| RA volume (mL) | 171 | 191 | 53 | — |
| cMRI | ||||
| RVEF (%) | 17 | |||
| SVI (mL/m2) | 29,7 | |||
| RVESVI (mL/m2) | 203 | |||
| Right heart catheterization | ||||
| mRAP (mmHg) | 12 | 12 | 3 | 7 |
| mPAP (mmHg) | 48 | 45 | 29 | 31 |
| PAP/SAP ratio | 0,56 | 0,40 | 0,23 | 0,32 |
| PCWP (mmHg) | 7 | 7 | 5 | 5 |
| PA saturation (%) | 40 | 63 | 70 | 77 |
| PVR* (UWood) | 21,84 | 10,11 | 5,59 | 3,91 |
| PVR index (UWood/m2) | 13,24 | 6,36 | 3,39 | 2,20 |
| SVR*(UWood) | 39,43 | 26,88 | 28,4 | 13,85 |
| PVR/SVR ratio | 0,55 | 0,38 | 0,20 | 0,28 |
| CO* (mL/min) | 1,88 | 3,76 | 4,30 | 6,64 |
| CI* (L/min/m2) | 1,14 | 2,37 | 2,61 | 3,73 |
CI – cardiac index; cMRI – cardiac magnetic resonance imaging; CO – cardiac output; FAC – fractional area change; mPAP – mean pulmonary artery pressure; mRAP – mean right atrial pressure; NTproBNP – N-terminal-pro-brain natriuretic peptide; PA – pulmonary artery; PCWP – post-capillary wedge pressure; PVR – pulmonary vascular resistance; RA – right atrium; RVEF – right ventricular ejection fraction; RVESVI – right ventricular end-systolic volume index; SAP – systolic arterial pressure; SVI – stroke volume index; SVR – systemic vascular resistance; TAPSE – tricuspid annular plane systolic excursion; TT – transthoracic; WHO-FC – world health organization functional class; 6MWT – 6 min walking test.
Due to very distal disease (mostly affecting the subsegmental branches and microvasculature), the patient was considered inoperable by an expert and high-volume surgical center and medical therapy alone was proposed. Combined PVT was started with oral Bosentan and intravenous Epoprostenol delivered by a Hickman line. Switch to subcutaneous Treprostinil was motivated by a total thrombosis of the internal jugular vein (despite adjusted oral anticoagulation by the I.N.R.). Gradual switch to oral Selexipag was needed due to refractory pain in the injection site.
After 3 months under optimized PVT (Riociguat 2.5 mg t.i.d., Bosentan 125 mg b.i.d. and Selexipag 1600 μg b.i.d.) the patient retained several increased-risk criteria. Therefore, he integrated a BPA program of seven sessions (a total of 11 segments, 31 vessels). Follow-up performed at 6 months showed significant clinical improvement (WHO class I and suspension of LTOT), as well as right heart dimension and function on echocardiogram and only residual pH on RHC (Table 1).
CTEPH treatment is very complex and requires management by a multidisciplinary team.1 PEA is the treatment of choice as it may cure the disease and effectively improve symptoms and survival (reported as >75% at 3 years).1–3 However, since its feasibility depends on the nature and location of the lesions, it is only possible for fewer than 60% of cases1,4,6 either because patients are technically inoperable (distal lesions not accessible by surgery) or because comorbidities impose a high surgical risk.1 Furthermore, approximately 50% of operated patients maintain residual PH or have recurrent disease.4 For inoperable patients, for those with residual or recurrent CTEPH after surgery and also for very distal disease, PVT is recommended to change the microvascular remodeling in response to obstruction, allowing symptom reduction and improvement in functional capacity.1,2,4–6 Although targeting endogenous nitric oxide, prostacyclin and endothelin pathways may help improve symptoms and haemodynamics in CTEPH patients, medical therapy alone does not cure the disease and these patients still have high mortality rates when compared to surgery.5,6 Hence, percutaneous reperfusion of segmental and subsegmental lesions with BPA emerged as an option for patients unsuitable to surgery or with residual/recurrent disease after PEA.1,2,5,6 Besides there is increasing evidence supporting its efficacy in improving symptoms, right heart function and haemodynamics,1,2,5–7 increasing experience and refinement of the technique significantly reduced rates of severe complications related to BPA (such as reperfusion pulmonary edema, bleeding or wire injuries),1 making its effectiveness comparable to surgery (2-year survival rate of 94.5% in a Japanese multicentric registry).7 These results are reflected in the most recent European guidelines on pH, that increased BPA class of recommendation from IIb to I.1
A careful consideration of the patient age and functional status as well as lesion location and response to medical therapy seems particularly relevant as this disease is associated with a high morbidity, mortality and health related costs. This case is intended to reflect how a multimodality approach was essential to improve this young patient's quality of life and prognosis.