Tyrosine kinase inhibitors (TKIs) are the mainstay of the current treatment of Philadelphia chromosome positive chronic myeloid leukemia (CML). Pulmonary complications associated with TKIs are more frequently reported with Dasatinib, particularly pleural effusion, although they can also be secondary to Bosutinib therapy.1-3 Here we present a case of a patient with CML treated with Bosutinib who developed a chylothorax.
A 68-year-old woman, non-smoker, with no history of significant comorbidities was diagnosed with chronic-phase CML in 2006. She was initially treated with Imatinib 400mg qd, achieving a complete molecular response. However, therapy was switched to Bosutinib 500mg qd in 2016, due to gastrointestinal intolerance. In 2021, she presented in the emergency department complaining of a one-month history of severe dyspnea (mMRC 3), dry cough and chest pain. On auscultation, there was a decrease in breath sounds on the right inferior lung field. There were no other abnormalities on physical examination.
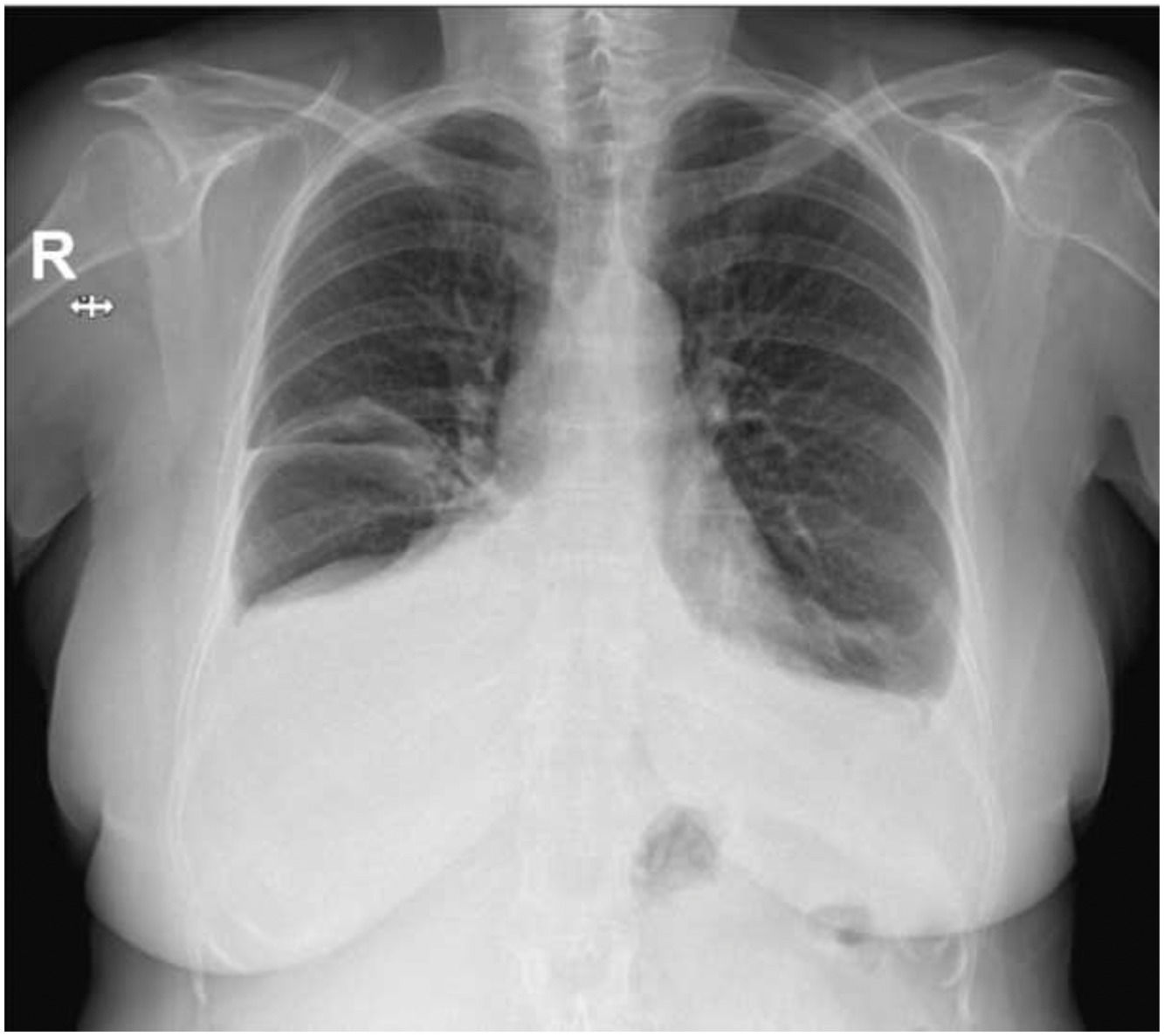
A chest radiograph revealed a small volume bilateral pleural effusion, which was larger on the right. A CT-Scan of the thorax was then performed showing a bilateral free-flowing pleural effusion, which was larger on the right, and a partial collapse of the right middle lobe with no clear obstructive cause. A flexible bronchoscopy provided better characterization with the finding of right middle bronchus tapering, allowing the progression of the bronchoscope. A bronchoalveolar lavage and brushing were performed in that bronchial segment, with no abnormalities found. An ultrasound-guided diagnostic thoracocentesis was performed, with the removal of 26 mL of pleural effusion with a hazy and milky appearance, classified as a lymphocytic predominant exudate. The pleural fluid was categorized as a chylothorax after the biochemical examination (pleural fluid triglyceride concentration of 375 mg/dL). The pleural fluid culture, immunophenotyping and cytology exam were negative. Liver function tests were normal.
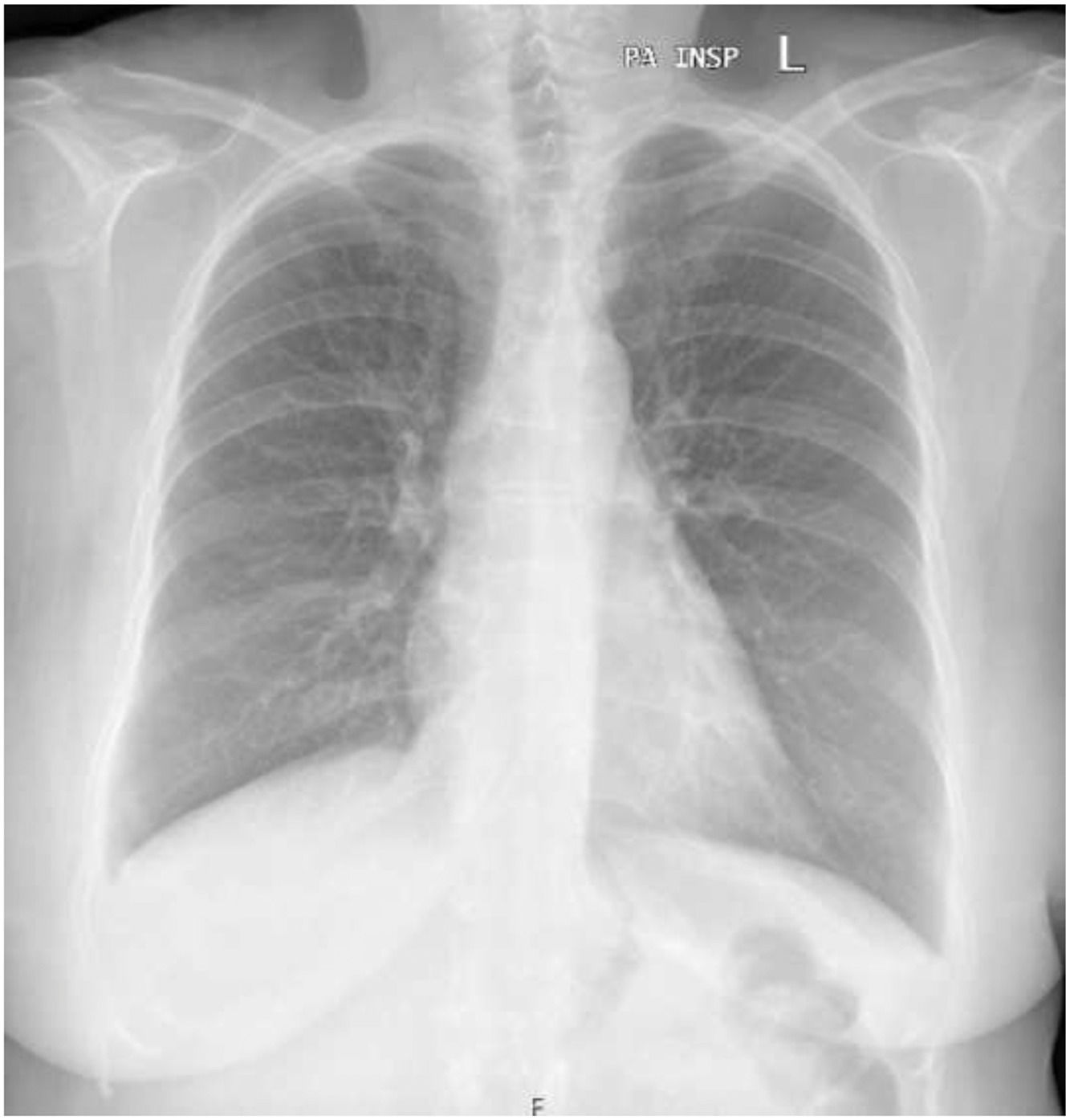
A clinical suspicion of a Bosutinib induced chylothorax was raised. Since all the criteria for stopping TKI were met, Bosutinib was withdrawn, with a complete resolution of the bilateral pleural effusion within five weeks, therefore confirming the diagnosis. Respiratory symptoms resolved within a week. The patient remains in complete molecular response after 6 months without TKI therapy.
Pulmonary toxicity is a common adverse effect of Dasatinib therapy, particularly pleural effusion, with a reported incidence as high as 39%.4 Although the risk decreases over time, it can occur throughout the whole treatment. Bosutinib has also been associated with pleural effusion, with a reported incidence around 5% in the first-line setting and up to 17% in later-line settings5. Known risk factors for Dasatinib-related pleural effusion include cardiac disease, arterial hypertension, pulmonary disease, hypercholesterolemia, autoimmune disease, advance phase CML and age older than 60 years and are thought to be the same for Bosutinib.4,6
Management of Dasatinib-related pleural effusion is based on its estimated size on chest x-ray and the severity of symptoms. Small, asymptomatic pleural effusions (< 500 mL) may only require close monitoring; if symptomatic, they can be managed with temporary TKI suspension and treatment can resume at the same or a lower dose.4,6 If the pleural effusion does not resolve with TKI suspension, diuretics or a short course of corticosteroids are options in stable patients. Severe pleural effusions which cause dyspnea may require thoracentesis.4,6 For recurrent pleural effusions, switching to another TKI should be considered depending on severity, so that CML treatment is not compromised with further dose reductions.6 There are no specific data regarding nutritional management of Dasatinib-related chylothorax, however there is a rationale to include a medium-chain triglyceride diet as an add-on strategy for large recurrent chylothorax. Similarly, no specific recommendations exist for the management of Bosutinib-related pleural effusions, but it seems reasonable to follow a similar strategy.
Although TKIs have revolutionized the treatment of patients with CML, there are clinically important pulmonary toxicities to be aware of. As far as we know, this is the first report of a Bosutinib-associated chylothorax. Other than older age, the patient had none of the risk factors known to be associated with Dasatinib-related pleural effusion. Therefore, and due to this infrequent presentation, a high clinical suspicion is required.
Patient consentWritten informed consent was obtained from the patient for publication of her clinical details and images.