Chronic Obstructive Pulmonary Disease (COPD) is associated with several co-morbidities, however their prevalence varies from one study to another.
AimTo determine the prevalence of several co-morbidities in patients with COPD severity score GOLD 4 (The Global Initiative for Chronic Obstructive Lung Disease, 2010) followed in ambulatory care, in a University Hospital.
MethodsA questionnaire was designed and carried out in order to characterize COPD and its co-morbidities. Clinical files were consulted in order to complete the data.
Results89 patients (87% male) with a mean age of 68 years old, of which 79% were ex-smokers, were included. The average value of FEV1 (forced expiratory volume in one second) was 38% of the expected values and all the patients presented chronic respiratory failure. Thirty-five patients (39%) were frequent exacerbators.
Thirty-seven patients (42%) had been hospitalized at least once due to exacerbation of their respiratory disease in the previous year, and 66 patients (74%) hospitalized in the previous five years.
Most of the patients (97%) presented at least one comorbidity, with an average of 4 co-morbidities per patient and an average Charlson index of 2.
The most frequent co-morbidities were cardiovascular diseases (69%), osteoarticular pathology (51%), erectile dysfunction (48%), sleep apnoea syndrome (43%) dyslipidaemia (35%), cataracts (31%), gastroesophageal reflux (29%) and diabetes (20%).
Frequent exacerbators presented an increased risk of having two or more co-morbidities (Odds Ratio of 5), as well as a higher prevalence of gastroesophageal reflux (p=0, 0006) and more hospitalizations in the last year and in the previous 5 years (p<0,001).
ConclusionThis study confirmed the high prevalence and the association of co-morbidities in patients with COPD severity score GOLD 4, thus justifying the need for a comprehensive and integrating therapeutic approach.
A Doença Pulmonar Obstrutiva Crónica (DPOC) está associada a várias comorbilidades, contudo a prevalência das mesmas varia entre os estudos.
ObjectivoDeterminar a prevalência das diversas comorbilidades em doentes com DPOC estádio IV do GOLD (The Global Initiative for Chronic Obstructive Lung Disease, 2010) seguidos em regime de ambulatório, num Hospital Universitário.
MétodosFoi concebido e aplicado um questionário com o objectivo de caracterizar a DPOC e suas comorbilidades. Os dados foram completados por consulta do processo clínico.
ResultadosForam incluídos 89 doentes (87% do género masculino), com média etária de 68 anos, 79% dos quais ex-fumadores. O valor de FEV1 (forced expiratory volume in one second) médio foi de 38% do previsto e todos os doentes apresentavam insuficiência respiratória crónica. Trinta e cinco doentes (39%) eram exacerbadores frequentes.
Trinta e sete doentes (42%) tinham apresentado pelo menos um internamento por exacerbação da sua doença respiratória no ano anterior e 66 doentes (74%) nos últimos 5 anos.
A maioria dos doentes (97%) apresentava pelo menos uma comorbilidade, com uma média de 4 comorbilidades por doente e um índice de Charlson médio de 2.
As comorbilidades mais frequentes foram doenças cardiovasculares (69%), patologia osteo-articular (51%), disfunção eréctil (48%), síndrome da apneia do sono (43%), dislipidémia (35%), cataratas (31%), refluxo gastro-esofágico (29%) e diabetes (20%).
Os exacerbadores frequentes apresentaram um risco aumentado de terem 2 ou mais comorbilidades (Odds Ratio de 5), bem como uma maior prevalência de refluxo gastro-esofágico (p=0,006) e um maior número de internamentos no último ano e nos 5 anos anteriores (p<0,001).
ConclusãoEste estudo confirmou a elevada prevalência e a associação de comorbilidades em doentes com DPOC GOLD estádio IV, justificando a necessidade de uma abordagem terapêutica abrangente e integradora.
Chronic Obstructive Pulmonary Disease (COPD) is characterized by a limitation of the airflow; it is a preventable and treatable disease. It is usually progressive and is associated to an enhanced inflammatory response to noxious particles or gases in the airways and lungs1. Exacerbations and co-morbidities contribute to the global severity in some patients, a fact that is responsible for the growing interest of the scientific community in characterizing the co-morbidities associated with COPD.
The physiopathological mechanism that is behind COPD and the diverse co-morbidities is not yet fully explained. The high prevalence of co-morbidities in patients with COPD is apparently due to numerous different factors and is related to age, to systemic effects of smoking and to adverse effects of some drugs.2,3 Moreover, COPD's systemic inflammation might also represent the mechanism between COPD and some co-morbidities 1,2,4
Co-morbidities that are most frequently associated to COPD are diabetes mellitus, osteoporosis, depression, and lung cancer.3–5.
The aim of this study was to determine the prevalence of multiple co-morbidities in patients with COPD severity score GOLD 4 followed in ambulatory care, and to determine a relationship between comorbidities, exacerbations and admissions.
Material and MethodsSamplingA cross-sectional study with a retrospective analysis was carried out. Patients were included consecutively between 15th of July of 2010 and 31st of December 2010.
As inclusion criteria, the patients had to have a diagnosis of very severe COPD, defined by the relationship between the forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) post bronchodilaton ≤ 0.70 and FEV1<to 30% of predicted or between 30 and 50% of what was expected, along with chronic respiratory failure (Severity Score GOLD 2010).1 The patients were followed in ambulatory care in the Elective Ventilation Ward, the Day Hospital for Patients with Respiratory Failure and Rehabilitation Unit.
Design of the StudyA questionnaire was designed to characterize COPD and the co-morbidities. It was conducted a medical doctor who interviewed all the patients either face-to-face or by telephone in order to characterize the disease and its co-morbidities. Data were completed by the consulting clinical files.
Several variables were evaluated in this survey, such as: demographic data, smoking habits, respiratory symptoms, degree of dyspnoea evaluated according to the Medical Research Council (MRC) Dyspnoea Scale.6, severity of the bronchial obstruction, co-morbidities, medication, exacerbations in the last year, number of hospital admissions in the last year and in the previous five years.
The co-morbidities records were completed by consulting clinical files. In order to determine the co-morbidities, the patients’ answers to the survey were considered and the information in the clinical file or what corresponded to the medication that the patient was taking. Co-morbidities were quantified using the Charlson index.8–10.
There is no consensual definition of comorbidity, and there is divergence resulting from difficulties in defining whether it concerns a single illness or two or more different illnesses.7 According to Rosin et al.7 comorbidity was defined as the presence of one or more diseases, excepting COPD, that could be caused by or be directly related to COPD, regardless of whether it was part of the natural spectrum of COPD. In this study, exacerbation was considered to be a worsening of the respiratory disease requiring treatment with antibiotics and/or systemic corticoids, as Hurst et al.11 defined it. According to the same authors, the term frequent exacerbators was applied to the patients that had two or more exacerbations in the last year.11
Statistical analysisAll the variables were tested according to their normal distribution through the frequency histogram and the Kolmogorov-Smirnov test. The difference between two averages was determined using the T-student or Mann-Whitney test.
When variables presented a normal distribution, T-student test was used. If, on the other hand, the variables ‘distribution was not normal, Mann-Whitney test was used. Categorical proportions and variables were analysed either with Chi-squared test or with Fisher test, when appropriate. Values p<0.05 were considered statistically significant.
Data analyses were done with the PASW software (version 18; SPSS inc., Chicago, IL, USA).
ResultsOur study included 89 patients, with a mean age of 68±9 years and mostly male (86.5%) (Table 1). The mean age in males was significantly higher than in females (69 years versus 61 years; p=0.007).
Demographic and Clinical Characteristics of the patients.
| Total (n=89) | Men (n=77) | Women (n=12) | p Value | |
| Age | 67.6±9.4 | 68.8±9.1 | 61.2±10.2 | 0.007* |
| Smoking status | 0.55 | |||
| - Smokers | 14 (15.7%) | 11 (14.3%) | 3 (25%) | |
| - Ex-smokers | 70 (78.7%) | 62 (80.5%) | 8 (66.7%) | |
| - Non-smokers | 5 (5.6%) | 4 (5.2%) | 1 (8.3%) | |
| Pack-years | 60±31.2 | 61.4±30.2 | 43±26.8 | 0.01* |
| Body mass index (Kg/m2) | 28.0±5.5 | 27.9±5.6 | 28.6±5.2 | 0.89 |
| Dyspnoea Index – MRC | 2.9±1.3 | 3.0±1.3 | 2.3±1.3 | 0.71 |
| Chronic cough and sputum | 43 (48.3%) | 36 (46.8%) | 7 (58.3%) | 0.46 |
| 38.1±13.9 | 36.5±12.4 | 49.2±19.1 | 0.055** | |
| FEV1% of predicted | ||||
| Respiratory failure | 0.75 | |||
| - Partial | 18 (20.2%) | 13 (16.9%) | 5 (41.7%) | |
| - Global | 71 (79.8%) | 64 (83.1%) | 7 (58.3%) | |
| Long-term oxygen therapy | 66 (74.2%) | 59 (76.6%) | 7 (58.3%) | 0.50 |
| BiPAP | 62 (69.7%) | 55 (71.4%) | 7 (58.3%) | 0.28 |
| Exacerbation in the last year | 54 (60.7%) | 47 (61%) | 7 (58.3%) | 0.55 |
| Number of exacerbations in the last year | 1.4±1.5 | 1.3±1.5 | 1.5±1.7 | 0.77 |
| Hospital admissions in the last year, due to exacerbation of respiratory disease | 37 (41.6%) | 31 (40.3%) | 6 (50%) | 0.55 |
| Hospital admissions in the last 5 years, due to exacerbation of respiratory disease | 66 (74.1%) | 54 (74%) | 9 (75%) | 1.00 |
| Frequent exacerbators | 35 (39.3%) | 30 (39%) | 5 (41.7%) | 1.00 |
Data presented in number (%) and average±SD. MRC: Medical Research Council; FEV1: Forced Expiratory Volume in the fist second; BiPAP: Bi-level Positive Airway Pressure.
In relation to smoking habits, 78.7% were ex-smokers and 15.7% were still smokers. The average smoking amount was 60 pack-years. Women presented a lower average smoking amount lower than men (43 pack-years versus 61 pack-years; p=0.01) (Table 1).
Seventy-five patients (84.3%) presented at least one respiratory symptom - cough, sputum, dyspnoea or wheezing – on most days and 48.3% presented chronic cough and sputum (Table 1).
All patients were on severity score GOLD 4, with an average FEV1 of 38.1% of the predicted values, and all presented chronic respiratory failure. Women had a higher average FEV1 than men (49.2% versus 37% of predicted values; p=0.055) (Table 1).
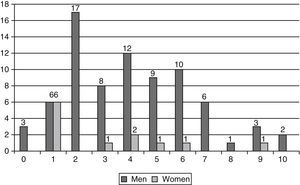
Most patients (86.6%) mentioned at least one comorbidity, with an average of 3.9 co-morbidities per patient, 83.1% had two or more co-morbidities (Table 2 and Fig. 1). The average Charlson index was 1.9 and 4.2 when adjusted to age (Table 2). Comparing the two genders, there was a larger proportion of men with at least two co-morbidities (88.3% of men versus 50% of women) (p=0.004) and a Charlson index of at least 2 (55.8% of men versus 25% of women) (p=0.047) (Table 2).
Description of the co-morbidities.
| Total(n=89) | Men(n=77) | (n=12) | p Value | |
| Co-morbidities | 3.9±2.4 | 4.1±2.4 | 3.1±2.6 | 0.11 |
| Charlson Index | 1.8±1.2 | 1.9±1.0 | 1.7±1.5 | 0.19 |
| Charlson Index age adjusted | 4±1.4 | 4.3±1.5 | 3.5±1.7 | 0.043* |
| Patients with ≥ 2 co-morbidities | 74 (83.1%) | 68 (88.3%) | 6 (50%) | 0.004** |
| Charlson Index ≥ 2 | 46 (51.7%) | 43 (55.8%) | 3 (25%) | 0.047*** |
| Cardiovascular Disease | 61 (68.5%) | 54 (70.1%) | 7 (58.3%) | 0.51 |
| - Arterial Hypertension | 51 (57.3%) | 46 (59.7%) | 5 (41.7%) | 0.24 |
| - Ischemic Heart Disease | 16 (18%) | 15 (19.5%) | 1 (8.3%) | 0.69 |
| - Arrhythmia | 16 (18%) | 15 (19.5%) | 1 (8.3%) | 0.69 |
| - Congestive Heart Failure | 4 (4.5%) | 4 (5.2%) | 0 | 1.0 |
| - Stroke | 4 (4.5%) | 4 (5.2%) | 0 | 1.0 |
| - Pulmonary Thromboembolism | 1 (1.1%) | 0 | 1 (8.3%) | 0.13 |
| Osteoarticular Pathology | 45 (50.6%) | 42 (54.5%) | 3 (25%) | 0.06 |
| Erectile Dysfunction | 37 (48.7%) | |||
| Sleep Apnoea Syndrome | 38 (42.7%) | 32 (41.6%) | 6 (50%) | 0.58 |
| Dyslipidaemia | 31 (34.8%) | 26 (33.8%) | 5 (41.7%) | 0.75 |
| Cataracts | 28 (31.5%) | 26 (33.8%) | 2 (16.7%) | 0.32 |
| Gastroesophageal Reflux | 26 (29.2%) | 22 (28.6%) | 4 (33.3%) | 0.74 |
| Diabetes mellitus | 18 (20.2%) | 16 (20.8%) | 2 (16.7%) | 1.00 |
| Depression | 14 (15.9%) | 9 (11.7%) | 5 (45.5%) | 0.013** |
| Cancer | 12 (13.5%) | 10 (13%) | 2 (16.7%) | |
| - Lung cancer | 3 (3.4%) | 3 (3.9%) | 0 | 1.00 |
| - Others | 9 (10.1%%) | 7 (9.1%) | 2 (16.7%) | 0.35 |
| Anaemia | 5 (5.6%) | 5 (6.5%) | 0 | 1.00 |
| Peptic Ulcer | 4 (4.5%) | 3 (3.9%) | 1 (8.3%) | 0.75 |
| Thyroid Pathology | 4 (4.5%) | 2 (2.6%) | 2 (16.7%) | 0.09 |
| Glaucoma | 3 (3.4%) | 2 (2.6%) | 1 (8.3%) | 0.36 |
| Cronic Renal desease | 2 (2.2%) | 2 (2.6%) | 0 | 1.00 |
Data presented in number (%) and average±SD.
As can be seen in Table 2, the most frequent co-morbidities were cardiovascular diseases (68.5%) and within this pathology the most prevalent was arterial hypertension (57.3%), followed by ischemic heart disease (18%), arrhythmia (18%) and congestive heart failure (4.5%). After cardiovascular pathology, the most prevalent diseases were: osteoarticular pathology (50.6%), erectile dysfunction (48%), sleep apnoea syndrome (42.7%), dyslipidaemia (34.8%), cataracts (31.5%), gastroesophageal reflux (29.2%), diabetes mellitus (29.2%), depression (15.7%) and cancer (13.5%). Of the patients with cancer, 3.4% had lung cancer and 10.1% had other cancers (larynx, bladder, liver, prostate, and breast).
With the exception of depression which was more prevalent in women (45.5% versus 11.7%; p=0.013), there were no statistically significant differences in the distribution of the others co-morbidities.
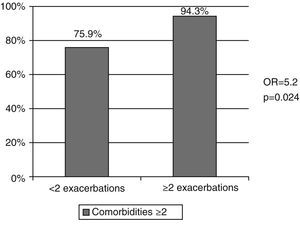
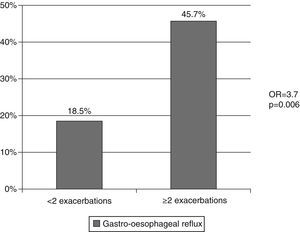
Fifty-four patients (60.7%) had presented at least one exacerbation of their respiratory disease in the last year, and of these, 49.6% had to be admitted to hospital care. Sixty-six patients (74.1%) had been admitted at least once due to worsening of COPD in the previous five years (Table 1). Thirty-five patients (39.3%) were classified as frequent exacerbators, meaning they had presented at least 2 exacerbations in the last year (Table 1) and presented an increased risk of having 2 or more co-morbidities (Odds ratio 5.2, p=0.024) (Fig. 2). An association between frequent exacerbators and gastroesophageal reflux was observed (Odd ratio 3.7; p=0.006) (Fig. 3). Of the 43 patients with cough and chronic sputum, 51.2% were frequent exacerbators, compared with 28.3% of the patients who did not present these symptoms (p=0.027). The frequent exacerbators had also more hospital admissions in the last year (1.2±1.2 versus 0.2±0.4) and in the previous 5 years (3.1±2.7 versus 1.4±1.2) (p<0.001).
DiscussionThe results of this study confirm that COPD is associated with several co-morbidities, as it has been documented in other studies.1,5,12–15.
A predominance of males was noted, a fact that may indicate that women in Portugal had smoked less in the 50's and 60's.
In this study we found several differences related to gender. Women presented less exposure to smoking with the same degree of obstruction and were younger, suggesting that they could be more susceptible to the effects of smoking. This observation has already been described by other authors.12,16.
Despite the fact that all patients were in GOLD stage 4, women presented a higher average FEV1 than men, which may be explained by the younger age of the women, reflecting the correlation between age and degree of obstruction, as it has been suggested by Fletcher and Peto.17.
The most frequent co-morbidities were cardiovascular diseases. The mechanism through which COPD can lead to cardiovascular events is not yet fully understood. Smoking is a common risk factor for both diseases. However, there seems to be a relationship between COPD and cardiovascular disease, which is independent of this risk factor.15 Studies showed that inflammation of airways can diffuse to systemic circulation, promoting a state of persistent systemic inflammation of low degree – a fact that together with other risk factors leads to the formation of plaques and to their rupture.18
It is important to highlight the prevalence of patients with COPD and lung carcinoma (3.4%). COPD is an independent risk factor for lung cancer, increasing risk by up to 2-5 times when compared to smokers without COPD.19,20 A direct correlation between the degree of airway obstruction and the risk of lung cancer was noted.21 Chronic inflammation may have a role in the pathogenesis of lung carcinoma, acting as a pro-oncogenic factor.19 Lung carcinoma was described as a cause of death in 7-38% of patients with COPD.3 The increased risk of lung carcinoma in patients with COPD, regardless of smoking habits, would justify the development of lung cancer screening programs for these patients.
The co-morbidities profile observed in this study was consistent with what has been reported in other studies. In a review of the literature, Chatila et al.3 demonstrated that cardiovascular diseases were present in 13-65% of patients with COPD, arterial hypertension in 18-52%, diabetes mellitus in 2-16%, arthritis in 22-70%, dyslipidaemia in 9-51% and cancer in 4-18%. These percentages are very similar to those noted in our study.
More recently, the Eclipse12 study compared the prevalence of different co-morbidities in COPD patients with a control group, it found that patients with COPD presented a higher prevalence of co-morbidities. The prevalence of stroke, heart failure, arrhythmia, gastroesophageal reflux and depression were similar to our study. However, we found a higher prevalence of ischemic heart disease (18% versus 9%) and diabetes mellitus (20.2% versus 10%).
Bárbara et al.22 performed a study with patients with GOLD COPD stage 2 to 4 and found that the most frequent co-morbidities were cardiovascular diseases (49%), followed by gastro-intestinal (20%) and metabolic diseases (16%).
In our study we only included patients with GOLD stage 4, however Eclipse12 and SAFE22 study included patients with GOLD stage 2 to 4 and we found a similar prevalence of co-morbidities. Eclipse study concluded that co-morbidities appeared to be independent of the degree of airflow limitation.
A possible explanation of the prevalance variability of the several co-morbidities in these studies could be the different methods and definitions used to evaluate co-morbidities.
In Eclipse study,12 compared to men, women with COPD were particularly susceptible to osteoporosis, inflammatory bowel diseases, gastroesophageal reflux and depression and were less likely to develop cardiovascular diseases and diabetes mellitus. In our study, we only found a statistically significant difference in the prevalence of depression, which was more frequent in women. A larger proportion of men had at least two co-morbidities and a Charlson index above 2. A possible explanation for this is the fact that the men were older than the women.
In our opinion, more studies are needed to understand the impact of COPD in several co-morbidities and to understand the influence of treatment of this pulmonary disease on co-morbidities.
In this study, 39.3% of the patients were frequent exacerbators. Several studies have demonstrated that exacerbations accelerate the decline of lung function that characterizes COPD.23,24 Eclipse12 study showed that there was a group of patients susceptible to exacerbations, regardless of the severity of bronchial obstruction and also that the phenotype of frequent exacerbators was associated with a history of gastroesophageal reflux, low quality of life and leukocytosis.
Microaspiration of gastric content and/or vagal irritation through gastroesophageal reflux may constitute an airways irritant and trigger a possible mechanism of exacerbation of COPD.25 In our study, as in Eclipse study11 and in that of Rascon-Aguilar et al,25 a statistically significant association between gastroesophageal reflux and frequent exacerbators was found. In our opinion, further studies are needed to evaluate the impact of this association and its treatment.
In this study, frequent exacerbators had an increased risk of having at least two co-morbidities (Odds ratio of 5). A possible explanation for this association was that the frequent exacerbators may have presented a greater systemic inflammation and thus, a higher number of co-morbidities. In order to prove this correlation and to understand the underlying physiopathological mechanisms, more studies are required. Burgel et al.26 described an association between the presence of cough and chronic sputum and frequent exacerbators in patients with COPD, just as we found in our study.
One of the limitations of our study was the fact that only GOLD stage 4 COPD patients were included, so our results cannot be generalized to the whole COPD population, even if, according to Eclipse12 study, co-morbidities were more frequently present in patients with COPD independently of GOLD score. In our case, not having a control group prevented us from evaluating the effect of age and other possible factors on the prevalence of co-morbidities.
ConclusionThis study confirmed a high prevalence of co-morbidities in patients with GOLD stage 4 COPD and their influence in exacerbations and hospital admissions, justifying a comprehensive and integrated approach.
It was shown that frequent exacerbators presented an increased risk of having at least two co-morbidities and a higher frequency of gastroesophageal reflux and hospital admissions in last year and in five previous years. The presence of chronic cough and sputum was associated with the occurrence of exacerbations of COPD.
Identification of the most frequent co-morbidities may be an important tool for improving our understanding of the complex relation between smoking habits, COPD and co-morbidities.
ETHICAL DISCLOSURESProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
AuthorshipVanda Areias collaborated in data collection and sampling, performed the statistical analysis and produced the first version of the article.
Susana Carreira and Marisa Anciães collaborated in data sampling and analysis and reviewed the article.
Paula Pinto and Cristina Bárbara designed and supervised all aspects of the study and reviewed it.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Areias V, Carreira S, Anciães M, Pinto P, Bárbara C, et al. Comorbilidades em doentes com doença pulmonar obstrutiva crónica estádio IV. Rev Port Pneumol. 2014;20:5–11.