Diabetes mellitus (DM) is a well-known risk factor for tuberculosis (TB). However, it is not known to what extent DM affects the outcome in patients with multidrug-resistant (MDR-TB) and extensively drug-resistant TB (XDR-TB) treated with second-line anti-TB drugs.
The objective of this study was to compare the microbiological evolution (sputum smear and culture conversion) and final outcomes of MDR/XDR-TB patients with and without DM, managed at the national TB reference centre in Mexico City.
ResultsNinety patients were enrolled between 2010 and 2015: 73 with MDR-TB (81.1%), 11 with pre-XDR-TB (e.g. MDR-TB with additional resistance to one injectable drug or a fluoroquinolone, 12.2%) and 6 (6.7%) with XDR-TB. Out of these, 49 (54.4%) had DM and 42 (86%) were undergoing insulin treatment.
No statistically significant differences were found in treatment outcomes comparing DM vs. non-DM MDR-TB cases: 18/32 (56.3%) of DM cases and 19/24 (79.2%) non DM patients achieved treatment success (p=0.07). The time to sputum smear and culture conversion was longer (although not statistically) in patients without DM, as follows: the mean (±SD) time to sputum smear conversion was 53.9 (±31.4) days in DM patients and 65.2 (±34.8) days in non-DM ones (p=0.15), while the time to culture conversion was 66.2 (±27.6) days for DM and 81.4 (±37.7) days for non-DM MDR-TB cases (p=0.06).
ConclusionsThe study results support the Mexican National TB programme to strengthen its collaboration with the DM programme, as an entry point for TB (and latent TB infection) screening and management.
Tuberculosis is among the leading causes of death from an infectious disease.1 In 2014, a global estimate of 450,000 cases of MDR-TB (multidrug-resistant tuberculosis, e.g. disease sustained by a Mycobacterium tuberculosis strain resistant to at least isoniazid and rifampicin) and XDR-TB (extensively drug-resistant TB, defined as MDR strain with additional resistance to a fluoroquinolone and one second-line injectable drug) producing over 150,000 deaths was made by the World Health Organization (WHO).1
Unfortunately, MDR-/XDR-TB achieves low treatment success rates as the available treatment regimens have limited efficacy, are toxic and expensive while requiring almost 2 years of treatment.2–4
Diabetes mellitus (DM), a well-known risk factor for TB, is the most important co-morbidity associated with TB in Mexico, its prevalence among all TB cases having increased from 5.8% in 2000 to 9.2% in 2012.5
As it is not clear to what extent DM affects treatment outcomes in MDR-/XDR-TB cases treated with second-line anti-TB drugs,6,7 we have compared the microbiological evolution (sputum smear and culture conversion) and final outcome of patients with confirmed MDR/XDR-TB with and without DM, managed at the national TB reference centre in Mexico City.
MethodsThe study was performed under a cooperative project involving the Mexican National Tuberculosis Programme, the ‘Instituto Nacional de Enfermedades Respiratorias’ (INER) in Mexico City, the International Union against Tuberculosis and Lung Disease, the Asociación Latinoamericana de Tórax and the European Respiratory Society. The study has been conducted at INER, the Mexican National TB Reference Centre, managing MDR-/XDR-TB cases from Mexico City and different Mexican States.
Patients underwent drug susceptibility testing (DST) at the quality-controlled INER microbiological laboratory by both Löwenstein–Jensen and the BACTEC-960 Mycobacterial Growth Indicator Tube (MGIT), for all first-line plus some second-line drugs (amikacin, kanamycin, ofloxacin, ethionamide). All patients underwent blood tests (including glycated haemoglobin) as part of the routine TB assessment prior to treatment or at least during the first week and at the end of treatment. DM was defined as a fasting blood glucose >126mg/dl in patients with no known DM history or self reported history; in patients with a previous DM history, evolution time and treatment type were also assessed. In addition, we performed blood biometry, blood chemistry, HbAC1, and thyroid stimulating hormone (TSH) at baseline and during final visits.
All patients were invited to initiate in-patient treatment (usually 2 weeks), in order to monitor adverse or allergic reactions and establish treatment tolerance; after discharge they were referred to the primary health centre (PHC). A strict directly observed therapy policy was applied both at hospital and PHC level.
Follow-up examinations were performed monthly during the intensive phase of treatment, and, then, every 2 months till treatment completion.
Treatments were individualized and based on DST as per WHO and Mexican Guidelines.8,9 They included at least 4 active drugs, one fluoroquinolone (ofloxacin, levofloxacin, moxifloxacin, presently classified as WHO Group A drug), one second-line injectable drug (amikacin, kanamycin, capreomycin, presently classified as WHO Group B drug), and, if necessary, one or more drugs from the former WHO group four (prothionamide, cycloserine, para-aminosalicylic acid) and five (linezolid, amoxicillin/clavulanate, and high-dose isoniazid), now included in Group C and D.3,4,8
The intensive phase of treatment (including the injectable drug) had a minimum duration of 6 months, the total treatment duration being 24 months.
Bivariate analysis was conducted with the variables as categorical or numeric (according to their distribution), and Kaplan–Meier analysis was performed to describe the time to sputum smear and culture conversion. All analyses were performed using the STATA statistical software package ver. 9.0 (StataCorp LP, College Station, TX, USA).
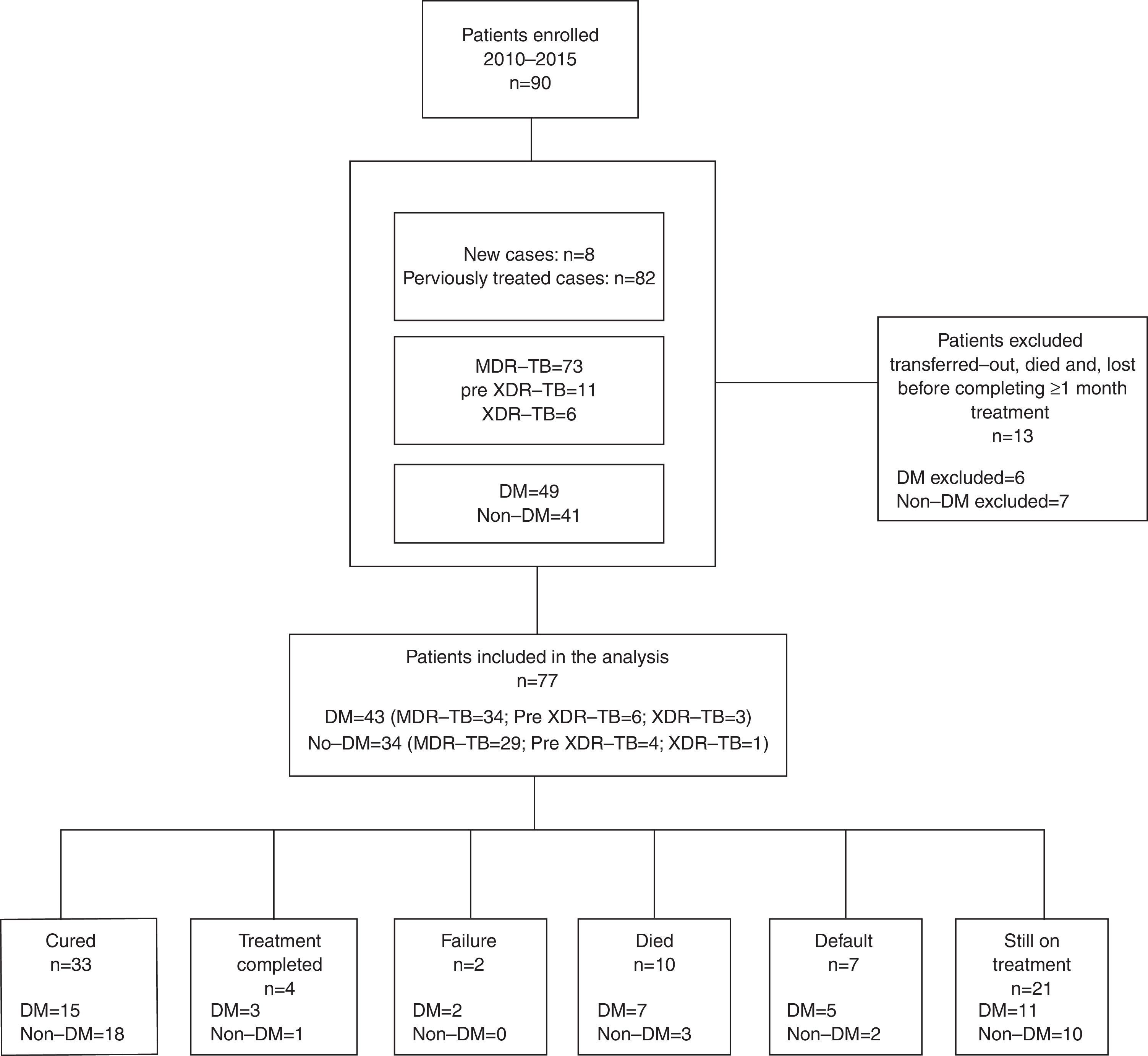
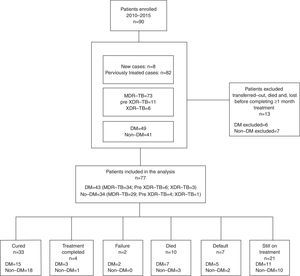
ResultsNinety patients were enrolled between 2010 and 2015 (Fig. 1): 73 with MDR-TB (81.1%), 11 with pre-XDR-TB (e.g. MDR-TB with additional resistance to one second line injectable drug or a fluoroquinolone, 12.2%) and 6 (6.7%) with XDR-TB. Out of these, 49 (54.4%) had type 2 DM, and 3 Human Immunodeficiency Virus (HIV) co-infection (3/90, 3.3%). Forty-two out of 49 (86%) MDR-TB patients affected by type 2 DM underwent insulin treatment at the moment treatment started.
The mean (±SD) glucose level of DM patients preceding anti-TB treatment was 210.8±102.5mg/dl, and glycated haemoglobin was 9.5±2.1%, while the levels after treatment completion in those who concluded treatment and among those who at least completed the intensive treatment phase at the moment of this report were: 175.3±84.3mg/dl and 8.8±2.3% (p=0.2 and p=0.7, respectively). No difference in the number of drugs to which the strain was resistant to (mean value±SD: 3.6±1.7 vs. 4.1±1.6; p=0.09) was identified among DM and non-DM patients; 82 out of 90 (91%) patients had history of at least one prior treatment; only 8 were treatment naïve, 5 having been in close contact with a MDR-TB patient.
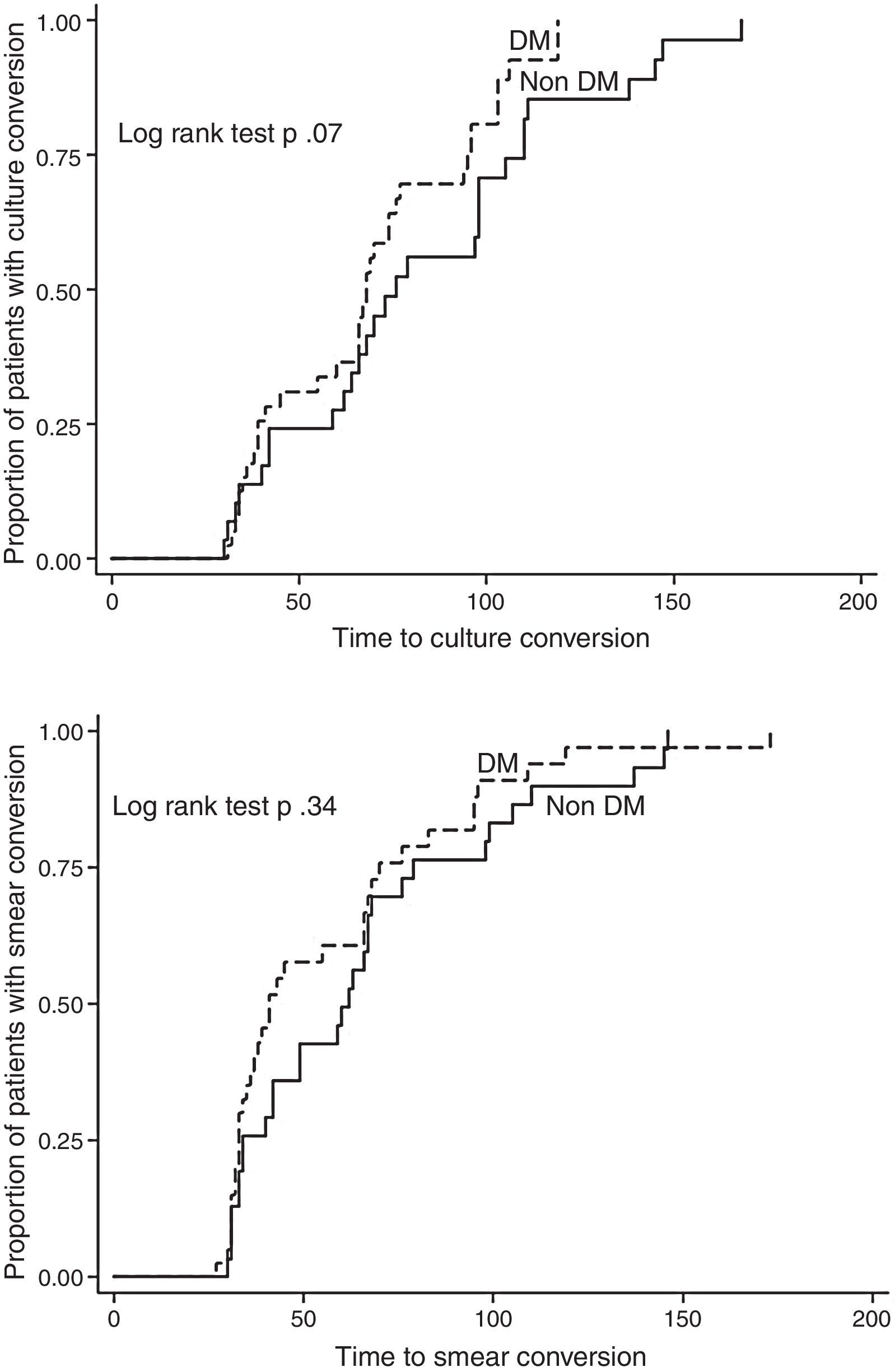
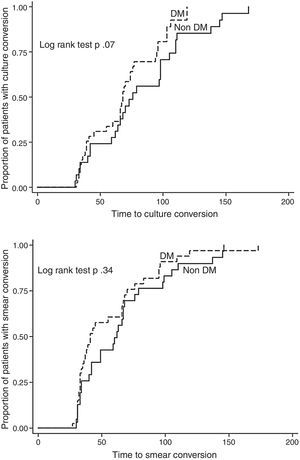
We excluded 13 patients (6 MDR, 4 pre-XDR, and 3 XDR) from the final analysis because they were transferred to another clinic, had died, or were lost to follow-up before completing at least 1 month of treatment, so that we could not evaluate either the effect of treatment or the outcome. Twenty-one patients (23.3%) are still on treatment (11 with DM and 10 without DM), while 15 have already concluded their intensive phase. Out of 56 patients for whom the outcome is available, 33 (59%) were cured according to the WHO definitions, 4 (7.1%) completed and 2 (3.6%) failed treatment, while 7 patients were lost to follow-up (12.5%) and 10 died (18%, 5 for reasons other than TB). No statistically significant differences were found in treatment outcomes comparing DM vs. non-DM MDR-TB cases: 18/32 (56.3%) of DM cases and 19/24 (79.2%) non-DM patients achieved treatment success (p=0.07). The time to sputum smear and culture conversion (Fig. 2) among DM and non-DM patients are as follows: the mean (±SD) time to sputum smear conversion was 53.9 (±31.4) days in DM patients and 65.2 (±34.8) days in non-DM ones (p=0.15), while the time to culture conversion was 66.2 (±27.6) days for DM and 81.4 (±37.7) days for non-DM MDR-TB cases (p=0.06). Although, it was longer in patients without DM, this was not statistically significant.
DiscussionThese preliminary study results suggest that although the bacteriological conversion was longer in non-DM patients (due to some delays in providing the sputum samples in this group), the final outcome was as expected higher in this group of non-DM patients although not reaching the statistical significance. However, it is important to underline that both success rates are higher than those available in the largest MDR-TB cohort published.2
However, for the first time, to the best our knowledge, these preliminary results compare the time to sputum smear and culture conversion in a Mexican cohort of MDR-TB cases with or without DM. Among the reasons which might explain the results (Fig. 2), we underline the role of improved treatment adherence: DM patients, given the disease chronicity, have been, in fact, trained to comply with the physician's prescriptions. However, the relatively limited sample size, the heterogeneous schedule for treatment monitoring (samples’ collection) and the delay in providing samples suggest caution in interpreting the data.
The overlap between TB (and MDR-TB) and DM supports the rationale for a programmatic collaboration. Additional information on the role that DM clinics can play as an entry point for TB (and latent TB infection) screening are necessary, particularly in countries (like Mexico) where the prevalence of both conditions is high.10
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors wish to thank the ERS (European Respiratory Society) and ALAT (Asociación Latinoamericana de Tórax) for the support to the SinTB Project, to which this study is part.