Chronic Obstructive Pulmonary Disease (COPD) is a serious pulmonary condition. Many patients experience exacerbations and some require Emergency Room visits and hospitalization. In Portugal, hospitalizations due to COPD between 2009 and 2016 decreased by 8%, but they still represented 8049 hospitalized patients in 2016. Appropriate management of COPD exacerbations presents a clinical challenge and, in order to guide therapy, it is important to identify the underlying cause; however, this is not possible in about a third of severe COPD exacerbations. There are several diagnostic tools that can be used to assess an exacerbation and its severity, which will in turn guide treatment, and prognostic scores should be used to predict the risk of future exacerbations. After an exacerbation is appropriately managed, a suitable discharge plan should be prepared. This should generally include reclassification of the patient according to GOLD criteria, optimization of pharmacological therapy, management of comorbidities, patient (or caregiver) education on the correct use of medications, referral to a Pulmonology Outpatient Clinic, if they are not already attending one, and a smoking cessation and respiratory rehabilitation program. In this paper, we will focus on the pharmacological strategies for the management of COPD exacerbations, risk stratification and a hospital discharge plan proposal.
Chronic Obstructive Pulmonary Disease (COPD) is a serious pulmonary condition, which is slowly progressive with systemic repercussions; it mainly affects people over 40 years old.1 However, COPD is preventable and treatable. Many patients experience COPD exacerbations and some of these require Emergency Room (ER) visits and hospitalizations. In Portugal, and although hospitalizations due to COPD between 2009 and 2016 have decreased by 8%, they still represented 8049 hospitalized patients in 2016. Hospitalizations of patients aged 80 years or more increased from 28.4% in 2005 to 38.0% in 2014, reflecting an aging population,2 with potentially more comorbidities.
Appropriate management of COPD exacerbations represents an important clinical challenge.3 In 70% to 80% of COPD exacerbations, the precipitant factor is a respiratory tract infection,4 but in about a third of severe exacerbations of COPD a cause cannot be identified,1 which hampers proper guidance of the therapeutic strategy. There are several diagnostic tools to assess an exacerbation and its severity, which will help in decisions like whether patient can be managed at home or in a primary care setting or if he/she should be referred to an ER and eventually hospitalized.1,5–7 The severity of an exacerbation will inform its treatment,1,7,8 and prognostic scores should be used to predict the risk of a future exacerbation. Three prognostic scores have been proposed based on biological and clinical characteristics of exacerbations: the BAP-65 score,9 the DeCOPD score9 and the score proposed by Roche et al.10,11
After an exacerbation is appropriately managed, a suitable discharge plan should be prepared. This will depend on the severity of the exacerbation, but should generally include reclassification of the patient according to the GOLD criteria,1 optimization of pharmacological therapy,1,4,8 management of comorbidities, patient (or home caregiver) education on the correct use of medications,1,8 referral to a Pulmonology Consultation if they are not already attending one, and a smoking cessation and pulmonary rehabilitation program.
ExacerbationsDefinition, causes and etiologyDefinitionCurrently, there is no exact or consistent definition of a COPD exacerbation. The definition of exacerbation in the 2016 GOLD update,12 “an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication”, was simplified in the GOLD 2017 document13 to “an acute worsening of respiratory symptoms that results in additional therapy”.
Causes and etiologySeveral factors that can lead to a worsening of symptoms have been identified, and in 70% to 80% of COPD exacerbation cases, the precipitant factor is a respiratory tract infection,4 either viral4,9,14,15 or bacterial,4,9,15 but in about a-third of severe exacerbations of COPD a cause cannot be identified.1
It is important to identify the underlying cause of an exacerbation as this will guide the therapeutic strategy.
ClassificationAs with the lack of definition of an exacerbation, there is no consensual classification system to assess the exacerbation severity, although some have been proposed.16 Some of these scores will be discussed further.
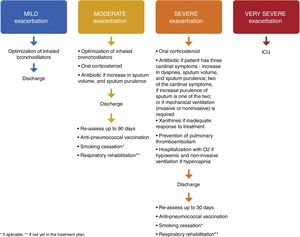
In mild exacerbations there is a worsening of symptoms which can be managed at home, with an increase in dosage of regular medications.1,6,17 Moderate exacerbations do not respond to an increased dosage of bronchodilators and therefore require treatment with systemic corticosteroids and/or antibiotics.1,6,17,18 Severe exacerbations require hospitalization or evaluation in the ER1,6,17,18 and have a severe impact on physical activity. Very severe exacerbations require admission to an Intensive Care Unit (ICU)1 and have a very severe impact on physical activity. Infectious exacerbations are characterized by increases in volume and purulence of the sputum associated with aggravated dyspnea and should be treated with antibiotics.1,8
Diagnostic toolsThe assessment of an exacerbation and its severity is based on the patient's medical history,1,6 e.g., airflow limitation, duration of worsening of symptoms and number of previous episodes (total/hospitalizations). Symptoms such as breathlessness, cough or sputum,7 oxygen saturation levels,7 new limitation of daily activities,6,7 clinical signs of severity such as use of accessory respiratory muscles,1,5 paradoxical chest wall movements,1,5 worsening or new onset central cyanosis,1,7 development of peripheral edema,1,7 hemodynamic instability,1 deteriorated mental status1,6,7 and comorbidities1 should all be assessed. Pulse oximetry should be performed on all patients.6 If a patient is referred to a hospital, arterial blood gases should be measured5,6,8,15,19–21 and a chest radiography should be done to exclude comorbidities and/or other pulmonary diseases.1,6,8,15,19 In these cases, it is also recommended that patients should have an ECG,1,6,19,20 whole blood count,1,6,8,20–22 and basic biochemical tests, including electrolyte concentrations,1,8,20,21 urea,8 glycemia1,20 and metabolic panel.6 Theophylline levels should be measured in patients on theophylline therapy at admission and blood cultures should be taken if the patient has fever.8 Culture of sputum samples is not recommended in routine practice, only if sputum is purulent,8 and the GOLD 2018 document recommends sputum culture and an antibiotic sensitivity test only if an infectious exacerbation does not respond to the empirical antibiotic treatment.1 Some authors mention eosinophilia blood count as an advisable procedure to guide COPD exacerbations therapy since it has been suggested that eosinophilic exacerbations may be more responsive to systemic steroids.1,15 Spirometry is not recommended during an exacerbation.1
If the exacerbation is severe and the patient hospitalized, brain natriuretic peptide and cardiac enzyme measurements levels should be considered, especially if the patient is not responding to conventional treatment.6 Also, pharyngeal swab or sputum should be tested for viruses and bacteria14,20,23 and serum C-reactive protein measured.14,20,24 Procalcitonin may guide antibiotic therapy since it has been suggested as a more specific marker for bacterial infections and that may be of value in deciding on antibiotics prescription.1 The Charlson comorbidity index,5,20,21,23 the modified Medical Research Council (mMRC) dyspnea scale,5,20,21,23 physical activity5 and general health5 should be assessed. The authors do not advise the use of COPD Assessment Test (CAT) score23 routinely in Portugal as it is not validated for the Portuguese population. If the patient is admitted to the ICU, besides the tests recommended in severe exacerbations, the Glasgow Coma Scale5 should be used, respiratory tract infections investigated25 and a hemoculture performed.24 According to the GOLD 2018 document only patients requiring non-invasive ventilation (NIV) or invasive ventilation (IV) should be hospitalized.1
Pharmacologic strategiesLABA+LAMAShort-acting inhaled β2 agonists (SABAs) and short-acting muscarinic antagonists (SAMAs) remain the mainstay in the treatment of symptoms and airflow obstruction during COPD exacerbations.1,4,6 Although at the time of publication of the GOLD 2018 document there were no clinical studies evaluating the usefulness of long-acting β2 agonists (LABA) or long-acting muscarinic antagonists (LAMA) in exacerbations, the recommendation is to continue this medication during the exacerbation or to start it as soon as possible before hospital discharge.1 The LABA+LAMA combination does have a documented benefit in the reduction of exacerbations when prescribed to patients in the stable phase of COPD,26 particularly the indacaterol/glycopyrronium combination as demonstrated in the SPARK27 and FLAME28 studies. Moreover, the recent FLAME study,28 the first prospective study evaluating blood eosinophilia as a biomarker of therapeutic response, showed that indacaterol/glycopyrronium demonstrated a significant improvement in lung function compared with salmeterol/fluticasone for all the cutoffs analyzed.29 A recent post hoc analysis of the WISDOM study identified a subgroup of patients – patients with ≥2 exacerbations and ≥400cells/μL – that seem to be at increased risk of exacerbation when discontinued from ICS.30 In fact, and according to the most recent version of the GOLD document,1 symptomatic patients in the stable phase of COPD and a history of ≥2 moderate exacerbations, or 1 with hospital admission, in the past year, may benefit from an ICS on top of LABA/LAMA. However, it is yet to be established whether blood eosinophils can be used as a biomarker to predict ICS efficacy in terms of exacerbation prevention, as suggested by the WISDOM post hoc analysis.1
Antibiotics, corticosteroids and xanthinesWhen treating an exacerbation adding oral or intravenous corticosteroids and/or antibiotics is recommended, depending on symptom severity and the presence of infection.1,4,6–8,31 Antibiotics should only be used for the treatment of infectious4,6,8,31 or severe exacerbations.31 The GOLD 2018 and NHS 2014 documents recommend antibiotics for patients with COPD exacerbations who have three cardinal symptoms – increase in dyspnea, sputum volume, and sputum purulence7 (Evidence B)1; have two of the cardinal symptoms, if increased purulence of sputum is one of the two symptoms7 (Evidence C)1; or require mechanical ventilation (invasive or non-invasive) (Evidence B).1
Antibiotics have been shown to reduce the risk of short-term mortality, treatment failure and sputum purulence, and a study in COPD patients with exacerbations requiring mechanical ventilation (invasive or non-invasive) indicated that not treating with antibiotics was associated with increased mortality and a greater incidence of secondary nosocomial pneumonia.1 A Cochrane review concluded that antibiotics for very severe COPD exacerbations showed wide and consistent beneficial effects across outcomes of patients admitted to an ICU,32 but this conclusion was based on data from a single study.32
The NHS protocol for management of COPD exacerbations in primary care states that bronchodilators and corticosteroids are the mainstay of exacerbation treatment.7 However, a systematic review of 19 COPD guidelines reported that the criteria for treating patients with antibiotics were largely based on an increase in respiratory symptoms, while systemic corticosteroids were often universally recommended for all patients with acute exacerbations.33 The authors also concluded that current COPD guidelines are of little help in identifying patients with acute exacerbations who are likely to benefit from treatment with systemic corticosteroids and antibiotics in primary care, which might contribute to overuse or inappropriate use of either treatment.
Some biomarkers have been suggested as useful for optimizing antibiotic treatment. The GOLD 2018 document1 does not recommend that CRP be used routinely but state that several studies have suggested that procalcitonin-guided antibiotic treatment reduces antibiotic exposure and side effects with the same clinical efficacy. This observation is corroborated by a Cochrane review demonstrating that procalcitonin can guide antibiotic therapy.32 In contrast, other authors reported that CRP might be a more valuable marker,34 and a real-life primary care study concluded that performing CRP rapid tests led general practitioners to prescribe fewer antibiotics than those who did not.35
For all patients, the choice of antibiotic should be guided by the local bacterial resistance pattern,1,8 the microbiology story of the patient and his/her risk factors.
Usually initial empirical treatment encompasses aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.1,8 However, the long-term use of macrolides may be associated with important side-effects and the risk of developing bacterial resistance.36 Sputum should be sent for culture (in the case of patients with frequent exacerbations, severe airflow limitation, and/or exacerbations requiring mechanical ventilation1), as gram-negative bacteria (e.g., Pseudomonas species) or resistant pathogens that are not sensitive to the above-mentioned antibiotics may be present.1
Although the most effective duration of treatment is still to be defined,32 the recommended length of antibiotic therapy is usually 5–7 days (Evidence D)1 but treatment duration will depend on the antibiotic used.
The management of exacerbations in primary care should include maximization of bronchodilator therapy and systemic corticosteroids if not contraindicated (30mg prednisolone) for 7 days.1,7,8 Therapy with oral prednisolone is equally as effective as intravenous administration.1 The GOLD 2018 document recommends a dose of 40mg prednisone per day for 5 days1 whilst NICE 2016 recommends a dose of 30mg for 7–14 days, and further recommends that a course of corticosteroid treatment should not be longer than 14 days as there is no advantage in prolonged therapy.8 The use of systemic corticosteroids in COPD exacerbations have been shown to shorten recovery time, improve lung function, improve oxygenation, decrease the risk of early relapse and treatment failure, and decrease the length of hospitalization.1
A meta-analysis confirmed that the rate of treatment success increased with systemic corticosteroids in comparison to usual care of COPD exacerbations. Corticosteroids seem to be beneficial to the whole population in terms of treatment success rate.37
Some studies suggest that corticosteroids may be less efficacious in treating acute COPD exacerbations in patients with lower levels of blood eosinophils.15,38
As for methylxanthines in the management of COPD exacerbations, current evidence does not support their use, given that the possible beneficial effects in lung function and clinical endpoints are modest and inconsistent, whilst adverse events are significant.1,4,6,31 Intravenous methylxanthines (theophylline or aminophylline) may be considered second-line therapy and used as an add-on when there is insufficient response. When using theophylline, it is necessary to monitor blood levels, side effects and potential drug interactions.8,31
Therapeutics – risk stratificationExacerbations of COPD may be classified as mild, moderate, severe6 and very severe. Very severe exacerbations require admission to the ICU, with invasive ventilation, and are outside the scope of this paper.
As previously mentioned, exacerbations of COPD are very heterogeneous making it particularly relevant to determine their etiology, pathology, severity and risk as all of these factors will have implications in the prognosis, pharmacological treatment and place of treatment.
In terms of pharmacological treatment and place of treatment, if exacerbations are mild and non-infectious,1,4,7,8,31 they may be treated at home with an increase in the dosage of maintenance bronchodilators.6,17 If the exacerbation is infectious4,8,31 an antibiotic should be given.1,7
Moderate exacerbations should be treated in the ER and the patient then discharged as these exacerbations do not require hospitalization, unless the hospitalization occurs for socioeconomic reasons. The dosage of maintenance bronchodilators should be increased6,17 and the patient been given an oral corticosteroid6,17,18 for 5 days.1,38,39 If the exacerbation is infectious4,8,31 an antibiotic should be given.1,7
In the case of a patient who has had a severe exacerbation, requiring hospitalization, the patient should be reclassified as a frequent exacerbator. Usually, hospitalization due to a severe exacerbation requires modification of inhaled maintenance treatment including O2 if the patient is hypoxemic and non-invasive ventilation if patient has hypercapnia, greater than 52cm H2O and/or acidemia,1,4,6,8 oral or intravenous corticosteroids (for 5 days)1,38,39 and antibiotic if infectious,1,7 xanthines if there is an inadequate response to treatment4,8,16,31 and prevention of pulmonary thromboembolism.
Discharge – action planPatients with mild exacerbations should be re-assessed after three months, with spirometry and a re-evaluation of the GOLD degree and, when appropriate, reclassification.
On discharge from a moderate exacerbation, bronchodilation should be optimized, anti-pneumococcal vaccination should be prescribed, and a smoking cessation and respiratory rehabilitation plan should be prepared.
On discharge after a severe exacerbation, optimal maintenance therapy1,4,8 with LABA, LAMA and ICS should be prescribed. Patients who have had an episode of respiratory failure should have satisfactory oximetry or arterial blood gas results before discharge. Patients (or home caregivers) should be given appropriate information to enable them to fully understand the correct use of medications, including inhalers and oxygen, and, if necessary, arrangements for follow-up and home care (such as visiting nurse, oxygen delivery, referral for other support) should be made. The patient, patient's caregiver and the physician should be confident that he or she can successfully manage the new treatment plan. When there is any doubt about the patient's capacity to manage his/her therapy, a formal activities of daily living assessment may be helpful.8 The GOLD 2018 document provides a list of discharge criteria.1 For patients who are hypoxemic during an exacerbation, arterial blood gases and/or pulse oximetry should be evaluated prior to hospital discharge and in the following 3 months. If the patient remains hypoxemic, long-term supplemental oxygen therapy may be required.1 Also, patients should be given clear instructions about when and how to stop their corticosteroid treatment.1,8 Concerning the need for individualized care, a Canadian study in which the patients were offered a post discharge phone call, a home visit and continued care concluded that although there was no reduction in 30- and 90-day readmission rates, a decrease in 90-day total mortality was seen. These data suggest that the individualized care undertaken in this study can impact COPD morbidity and mortality after an acute exacerbation.40 All patients who have had a severe exacerbation should be re-assessed 4–6 weeks after discharge from hospital,1 given an anti-pneumococcal vaccination prescription, and a smoking cessation and respiratory rehabilitation plan should be prepared – Fig. 1.
The authors propose that the patient should be prescribed an anti-pneumoccocal vaccine 10 to 20 days after discharge from the ER or Hospital.
During the follow-up consultation (three months for moderate exacerbations and 4–6 weeks for severe exacerbations), spirometry and arterial blood gases should be measured. Symptoms, correct use of inhaled therapy and adequate management of comorbidities should be re-assessed. Pharmacological treatment should be optimized. The smoking cessation and respiratory rehabilitation plan should be evaluated. A new follow-up consultation should be scheduled within the next 30–60 days.
ConclusionsIdentification of the underlying cause of COPD exacerbations and assessment of their severity is fundamental to guiding treatment. After an exacerbation is appropriately managed, a suitable discharge plan that will depend on its severity should be prepared. A proper discharge plan will decrease symptom burden, contribute to a faster recovery, increase the patient's quality of life, and prevent or delay future exacerbations. Referral to a Pulmonology Consultation if the patient is not already attending one is of the utmost importance.
Conflicts of interestAR declares having received speaking fees from AstraZeneca, Boehringer Ingelheim, Novartis, Bial, Medinfar, Mundipharma, Menarini, Grifols, Mylan, Tecnifar, Teva and cslbehring. CA declares having received speaking fees from AstraZeneca, Pfizer, Novartis and Mundipharma. SF declares no conflicts of interest. JF declares speaking fees from AstraZeneca, Boehringer Ingelheim, Diater, Inmunotek, Menarini, Mundipharma, Mylan, Tecnifar and TEVA, and participating in advisory boards of Bial, GSK and Novartis. MD declares having received fees for talks from AstraZeneca, Boehringher Ingelheim, Bial, GSK, Menarini and Novartis and for participation in advisory boards of Bial, GSK and Novartis. CRC declares speaking fees from Boehringer Ingelheim, Roche, Novartis, AstraZeneca, Pfizer vaccines, Teva, Menarini, Medinfar and Tecnifar, and participating in advisory boards of Boehringer Ingelheim, Roche, Novartis, GSK, AstraZeneca and Pfizer vaccines.
Funding for this paper was provided by Novartis Portugal. Funding was used to access all necessary scientific bibliography and cover meeting expenses. Novartis Portugal had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.