The aim of this study was to compare the effects of pulmonary rehabilitation (PR) on six-minute walk test (6mWT) between chronic obstructive pulmonary disease (COPD) patients with moderate or severe carbon monoxide diffusion defects. We also evaluated dyspnea sensation, pulmonary functions, blood gases analysis, quality of life parameters and psychological symptoms in both groups before and after pulmonary rehabilitation.
MethodsPatients with COPD underwent a comprehensive 8-week out-patient PR program participated in this study. Patients grouped according to diffusion capacity as moderate or severe. Outcome measures were exercise capacity (6mWT), dyspnea sensation, pulmonary function tests, blood gases analysis, quality of life (QoL) and psychological symptoms.
ResultsA total of 68 patients enrolled in the study. Thirty-two (47%) of them had moderate diffusion defect [TlCO; 52 (47–61)mmol/kPa] and 36 (53%) of them had severe diffusion defect [TlCO; 29 (22–34)mmol/kPa]. At the end of the program, PaO2 (p=0.001), Modified Medical Research Council dyspnea scale (p=0.001), 6mWT (p<0.001) and quality of life parameters improved significantly in both groups (p<0.05). Also the improvement in DlCO (p=0.04) value and FEV1% (p=0.01) reached a statistically significant level in patients with severe diffusion defect. When comparing changes between groups, dyspnea reduced significantly in patients with severe diffusion defect (p=0.04).
ConclusionPulmonary rehabilitation improves oxygenation, severity of dyspnea, exercise capacity and quality of life independent of level of carbon monoxide diffusion capacity in patents with COPD. Furthermore pulmonary rehabilitation may improve DlCO values in COPD patients with severe diffusion defect.
Chronic obstructive pulmonary disease (COPD) is defined by airflow limitation and is a complex pathological condition. COPD is associated with an important reduction in physical activity that contributes to the patient's disability and poor health-related quality of life. Pulmonary rehabilitation (PR) is aimed to eliminate or at least attenuate these difficulties.1,2 Therefore, PR has been recommended as an integral part of management for patients with COPD.3,4 However, the responses to PR may vary significantly among individuals. Although there are many studies mentioning changes of FEV1, FVC, FEV1/FVC after PR, in some studies, significant changes in forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and FEV1/FVC values were not detected after PR.5–8
The diffusing capacity for carbon monoxide (DlCO) is a common and clinically useful test that provides a quantitative measure of gas transfer in the lungs.9 The decrease in DlCO, one of the first signs of disease progression, can point out the arterial O2 desaturation during exercise. For COPD patients with low DlCO values pose a high risk for poor survival.10 Although PR is known to have many effects on functional outcomes of COPD patients, there is not enough information about the diffusion capacity in terms of PR outcome.2 Also in a recent study authors suggest that diffusing capacity was the strongest predictor of exercise capacity in all subjects with COPD.11 Therefore can CO diffusion capacity be used to evaluate which patient will benefit more from PR program? Moreover will there be a significant change in CO diffusion capacity after PR program in patients with COPD?
The aim of our study was to compare the results of PR program on exercise capacity (6mWT) between COPD patients with moderate and severe diffusion defect detected by DlCO. Our secondary aims were comparing the results of the program on arterial blood gas analysis, dyspnea sensation, exercise capacity, quality of life and psychological symptoms between two groups.
MethodsWe conducted a prospective cohort study to compare the effectiveness of exercise training in COPD patients with moderate and severe diffusion defect. The study was approved by the local institutional review board. Patients included in the study completed an informed written consent form.
Subject selectionWe recruited COPD patients diagnosed according to Global initiative for Chronic Obstructive Lung Disease (GOLD) definition, stable from exacerbations (with no worsening of respiratory symptoms, no increase in the use of rescue medication, and no unscheduled visits due to COPD worsening) for at least 4 weeks. All patients were suffering from dyspnea, reduced exercise tolerance and limitation of daily living activities. The recruitment criteria included a minimum age of 40 years old, a history of 10 or more pack-years of smoking, a FEV1 of less than 80% of the predicted value after bronchodilator use and a ratio of FEV1 to FVC of 0.7 or less after bronchodilator use.12 The condition of the patients was graded according to the stages of disease defined by the GOLD.13 The patients’ self-reported respiratory symptoms, medications, smoking history, and coexisting medical conditions were documented at the beginning of the study. Comorbid diagnoses were established using the clinical history and physical examination findings during the visit and were supported by a review of the available medical records. We excluded patients who were suffering from acute exacerbation, history of other lung diseases, (pneumoconiosis, pulmonary tuberculosis, interstitial lung disease); and orthopedic, neurologic, or cardiovascular impairment that might render the subject incapable of completing the exercise training. Also subjects with lack of motivation, poor compliance (not attending the program more than 2 times) or having transport problems were excluded from the study. We grouped patients; those with diffusion capacity between 41 and 60% of predicted as moderate (group 1) and under 40% of predicted as severe (group 2) diffusion defect.14
Measurement of pulmonary parameters and questionnairesAll patients underwent cardiac and respiratory system examinations and were evaluated by chest X-rays and blood gases analysis. Pulmonary functions were assessed by measuring body plethysmography (Zan 500, Germany) and carbon monoxide diffusing capacity test (Zan 300, Germany). The DLCO maneuver begins with a full exhalation to residual volume (RV), the mouthpiece is then connected to the test gas (0.3 percent carbon monoxide [CO], 10 percent helium), and the subject inhales rapidly to total lung capacity. Following a 10s breath hold, the subject exhales quickly and completely to RV. An alveolar sample of the exhaled gas is then analyzed for calculation of the dilution of helium and the uptake of CO. Dyspnea was assessed by Modified Medical Research Council (MMRC) dyspnea scale and modified BORG scales.3 Quality of life was assessed using disease specific St. George Respiratory Questionnaire and SF-36 health related quality of life questionnaire.15,16 Psychological symptoms were assessed by Hospital Anxiety and Depression Questionnaires.17,18 6-Minute walking test was used which was defined by American Thoracic Society (ATS) standards.19 All measurements were assessed at admission and at the end of the PR.
Pulmonary rehabilitation parametersPatients underwent an 8-week hospital based out-patient pulmonary rehabilitation program twice a week in our hospital's Pulmonary Rehabilitation Unit. Pulmonary rehabilitation was completely tailored to suit the needs of the individual. PR program consists of education, supervised exercise training, nutritional intervention and psychological counseling, if needed. Exercises were chosen for each patient for their ability to tolerate exercise and their disease severity. Exercises included; breathing exercises, treadmill (at least 15minutes) and cycle training (at least 15minutes), peripheral muscle training, and stretching exercises. Also we informed patients about medication advices, bronchial hygiene techniques, energy conservation, relaxation techniques for reducing dyspnea and home exercises.3 After respiratory physiotherapy education, upper and lower extremity stretching and strengthening exercises were performed. All strengthening exercises were started without a weight. During PR program according to the BORG scale4–6 half a kilogram weight is added in every 4 periods of exercises. The treadmill and bicycle/arm ergometer were used for aerobic exercises. We calculated both workloads for cycling and walking speed for treadmill from six minutes’ walk test.4 Treadmill walking speed is calculated by 80% of average 6MWT speed using formula: (6mWT distance×10)/1000km/h. Cycling workload was calculated with the formula (Watt=103.217+(30.500×Sex)+(−1.613×age)+[(0.002×distance×weight)] sex; male=1 female=0). Patients were trained at 60–90% of maximum heart rate. Also we used BORG dyspnea scores for regulation of exercise.3–20 Exercise intensity increased according to the patient progress. During exercise we used pulse oximetry to supervise patients and if the SpO2 fell below 90% oxygen supplementation was provided.
Statistical analysisWe performed statistical analyses using the SPSS 17.0 (Statistical Package for the Social Sciences, Chicago, Illinois). Descriptive statistics were performed for all the recorded variables. The normality of the data was evaluated by Kolmogorov Smirnow test. We used Mann Whitney-U test to compare baseline characteristics and changing outcomes as shown Δ values before and after PR. We used Wilcoxon test to compare variables between before and after PR. p<0.05 value is considered as significant.
ResultsDemographic dataA total of 68 patients (61 men %90) participated in this study. Thirty-two (47%) patients had moderate diffusion defect [TlCO; 57 (47–61)mmol/kPa] (group 1) and 36 (53%) patients had severe diffusion defect [TlCO; 29 (22–34)mmol/kPa] (group 2). All patients had smoking history. Groups were similar in terms of age, gender and cigarette consumption (p>0.05). Body mass index (BMI) was significantly lower in patients with severe diffusion defect (p<0.05).
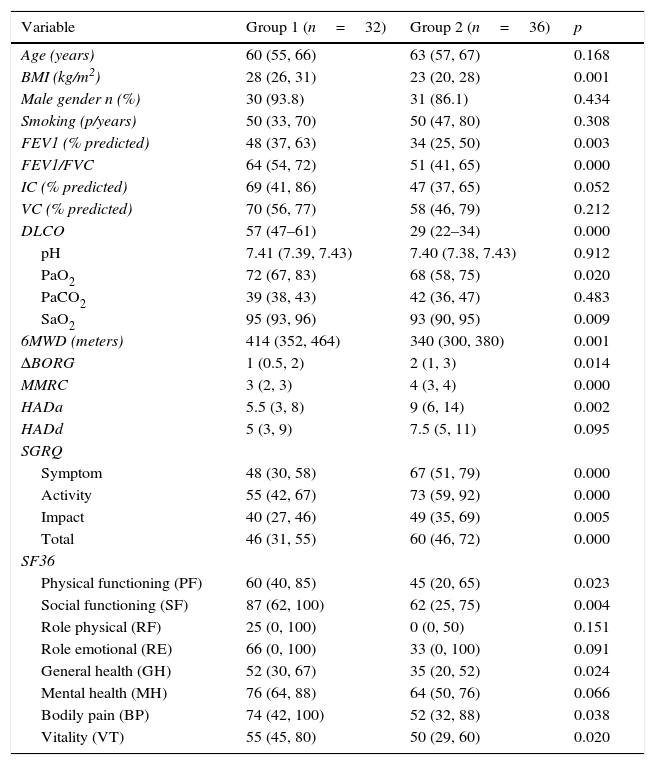
Initial measurements before PRWhen comparing initial measurements; exercise capacity, quality of life, FEV1, FEV1/FVC, PaO2, SaO2 were significantly lower in group 2 (p<0.05) before PR in comparison with group 1 (Table 1). Also dyspnea sensation and anxiety levels were significantly higher in group 2 compared with group 1 before PR.
Baseline demographic and clinical features of patients.
| Variable | Group 1 (n=32) | Group 2 (n=36) | p |
|---|---|---|---|
| Age (years) | 60 (55, 66) | 63 (57, 67) | 0.168 |
| BMI (kg/m2) | 28 (26, 31) | 23 (20, 28) | 0.001 |
| Male gender n (%) | 30 (93.8) | 31 (86.1) | 0.434 |
| Smoking (p/years) | 50 (33, 70) | 50 (47, 80) | 0.308 |
| FEV1 (% predicted) | 48 (37, 63) | 34 (25, 50) | 0.003 |
| FEV1/FVC | 64 (54, 72) | 51 (41, 65) | 0.000 |
| IC (% predicted) | 69 (41, 86) | 47 (37, 65) | 0.052 |
| VC (% predicted) | 70 (56, 77) | 58 (46, 79) | 0.212 |
| DLCO | 57 (47–61) | 29 (22–34) | 0.000 |
| pH | 7.41 (7.39, 7.43) | 7.40 (7.38, 7.43) | 0.912 |
| PaO2 | 72 (67, 83) | 68 (58, 75) | 0.020 |
| PaCO2 | 39 (38, 43) | 42 (36, 47) | 0.483 |
| SaO2 | 95 (93, 96) | 93 (90, 95) | 0.009 |
| 6MWD (meters) | 414 (352, 464) | 340 (300, 380) | 0.001 |
| ΔBORG | 1 (0.5, 2) | 2 (1, 3) | 0.014 |
| MMRC | 3 (2, 3) | 4 (3, 4) | 0.000 |
| HADa | 5.5 (3, 8) | 9 (6, 14) | 0.002 |
| HADd | 5 (3, 9) | 7.5 (5, 11) | 0.095 |
| SGRQ | |||
| Symptom | 48 (30, 58) | 67 (51, 79) | 0.000 |
| Activity | 55 (42, 67) | 73 (59, 92) | 0.000 |
| Impact | 40 (27, 46) | 49 (35, 69) | 0.005 |
| Total | 46 (31, 55) | 60 (46, 72) | 0.000 |
| SF36 | |||
| Physical functioning (PF) | 60 (40, 85) | 45 (20, 65) | 0.023 |
| Social functioning (SF) | 87 (62, 100) | 62 (25, 75) | 0.004 |
| Role physical (RF) | 25 (0, 100) | 0 (0, 50) | 0.151 |
| Role emotional (RE) | 66 (0, 100) | 33 (0, 100) | 0.091 |
| General health (GH) | 52 (30, 67) | 35 (20, 52) | 0.024 |
| Mental health (MH) | 76 (64, 88) | 64 (50, 76) | 0.066 |
| Bodily pain (BP) | 74 (42, 100) | 52 (32, 88) | 0.038 |
| Vitality (VT) | 55 (45, 80) | 50 (29, 60) | 0.020 |
Data are expressed as median (interquartile range) or %, BMI: body mass index, FEV1: forced expiratory volume in the 1s, FVC: forced vital capacity, IC: inspiratory capacity, VC: vital capacity, PaCO2: partial arterial carbon dioxide pressure. SaO2: arterial oxygen saturation, 6MWD: six minutes walk distance, MMRC: Medical Research Council Dyspnea Scale, HAD: Hospital Anxiety and Depression Scale, SGRQ: St. George Respiratory Questionnaire, SF-36: Short-Form Health Survey.
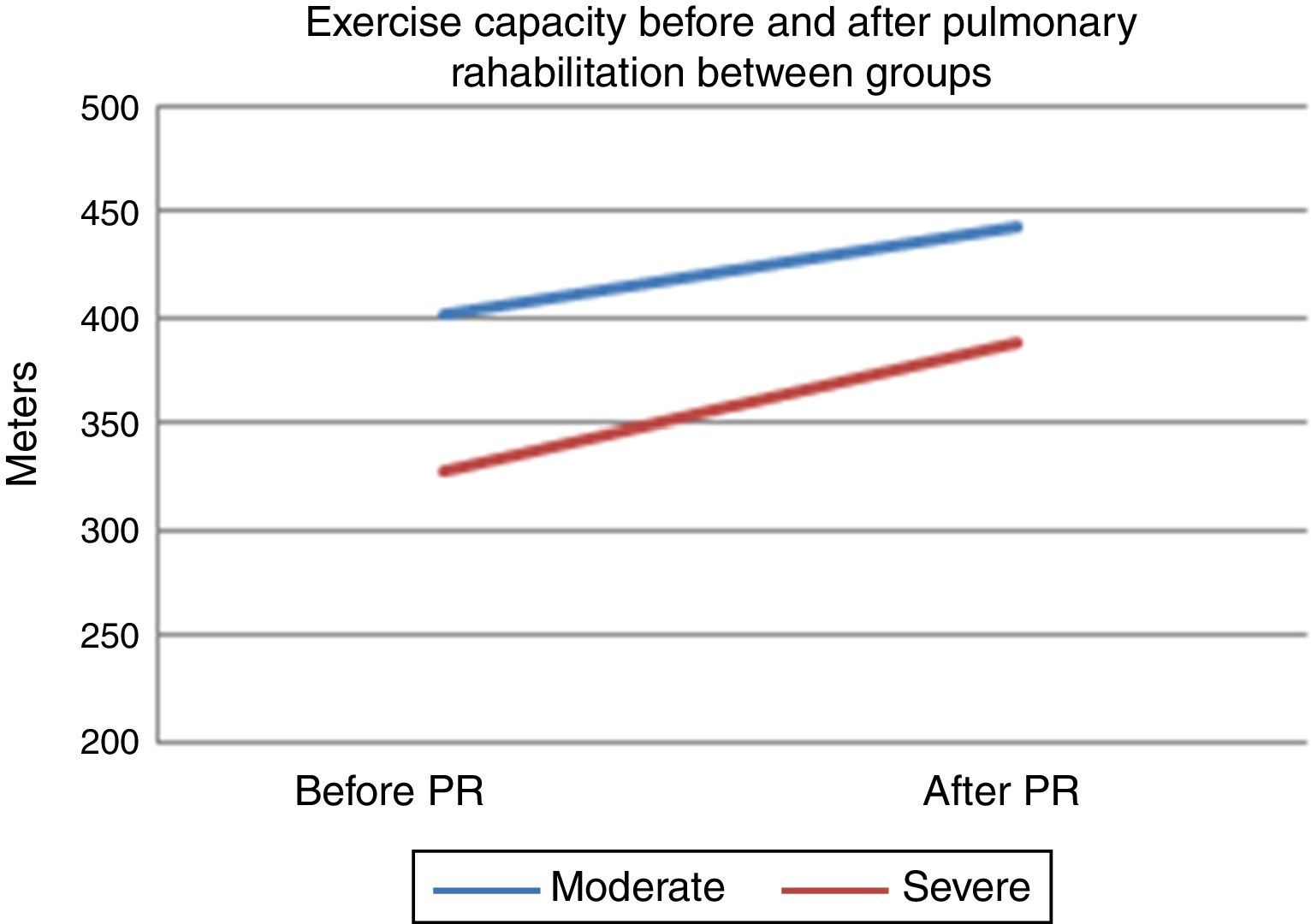
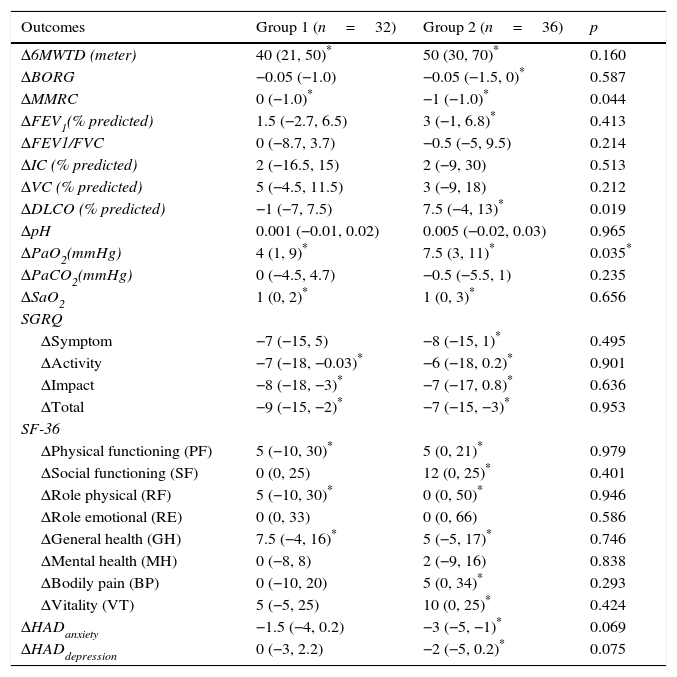
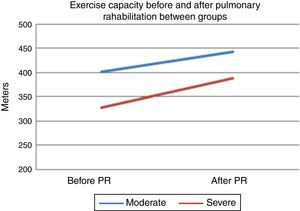
After PR program there was a statistically significant increase in 6mWT in both groups (401–443m for group 1, 328–388m for group 2, p<0.001, both) (Fig. 1). In both groups PaO2 significantly increased after rehabilitation (p<0.05, both). After rehabilitation, significant improvement recorded in dyspnea sensation (mMRC scores) and SGRQ scores (−9 for group 1, −7 for group 2) in both groups (p<0.05) (Table 2). There was no significant difference in both groups in term of ΔFEV1/FVC, ΔIC, ΔVC, ΔpH, ΔPaCO2, ΔRole emotional scores (SF-36) and Δmental health score (SF-36) after PR program.
Comparison of changes in exercise capacity, dyspnea, pulmonary functions, blood gas analyses, quality of life and psychological symptoms between two groups.
| Outcomes | Group 1 (n=32) | Group 2 (n=36) | p |
|---|---|---|---|
| Δ6MWTD (meter) | 40 (21, 50)* | 50 (30, 70)* | 0.160 |
| ΔBORG | −0.05 (−1.0) | −0.05 (−1.5, 0)* | 0.587 |
| ΔMMRC | 0 (−1.0)* | −1 (−1.0)* | 0.044 |
| ΔFEV1(% predicted) | 1.5 (−2.7, 6.5) | 3 (−1, 6.8)* | 0.413 |
| ΔFEV1/FVC | 0 (−8.7, 3.7) | −0.5 (−5, 9.5) | 0.214 |
| ΔIC (% predicted) | 2 (−16.5, 15) | 2 (−9, 30) | 0.513 |
| ΔVC (% predicted) | 5 (−4.5, 11.5) | 3 (−9, 18) | 0.212 |
| ΔDLCO (% predicted) | −1 (−7, 7.5) | 7.5 (−4, 13)* | 0.019 |
| ΔpH | 0.001 (−0.01, 0.02) | 0.005 (−0.02, 0.03) | 0.965 |
| ΔPaO2(mmHg) | 4 (1, 9)* | 7.5 (3, 11)* | 0.035* |
| ΔPaCO2(mmHg) | 0 (−4.5, 4.7) | −0.5 (−5.5, 1) | 0.235 |
| ΔSaO2 | 1 (0, 2)* | 1 (0, 3)* | 0.656 |
| SGRQ | |||
| ΔSymptom | −7 (−15, 5) | −8 (−15, 1)* | 0.495 |
| ΔActivity | −7 (−18, −0.03)* | −6 (−18, 0.2)* | 0.901 |
| ΔImpact | −8 (−18, −3)* | −7 (−17, 0.8)* | 0.636 |
| ΔTotal | −9 (−15, −2)* | −7 (−15, −3)* | 0.953 |
| SF-36 | |||
| ΔPhysical functioning (PF) | 5 (−10, 30)* | 5 (0, 21)* | 0.979 |
| ΔSocial functioning (SF) | 0 (0, 25) | 12 (0, 25)* | 0.401 |
| ΔRole physical (RF) | 5 (−10, 30)* | 0 (0, 50)* | 0.946 |
| ΔRole emotional (RE) | 0 (0, 33) | 0 (0, 66) | 0.586 |
| ΔGeneral health (GH) | 7.5 (−4, 16)* | 5 (−5, 17)* | 0.746 |
| ΔMental health (MH) | 0 (−8, 8) | 2 (−9, 16) | 0.838 |
| ΔBodily pain (BP) | 0 (−10, 20) | 5 (0, 34)* | 0.293 |
| ΔVitality (VT) | 5 (−5, 25) | 10 (0, 25)* | 0.424 |
| ΔHADanxiety | −1.5 (−4, 0.2) | −3 (−5, −1)* | 0.069 |
| ΔHADdepression | 0 (−3, 2.2) | −2 (−5, 0.2)* | 0.075 |
Data are expressed as median (interquartile range), Results are shown as change between post-treatment and baseline levels (Δ values).
p<0.05 for within group change, 6MWD: six minutes walk distance, MMRC: Medical Research Council Dyspnea Scale, FEV1: forced expiratory volume in the 1s, FVC: forced vital capacity, IC: inspiratory capacity, VC: vital capacity, DLCO: carbon monoxide diffusing capacity. PaO2: partial arterial oxygen pressure, PaCO2: partial arterial carbondioxide pressure. SaO2: arterial oxygen saturation, SGRQ: St. George Respiratory Questionnaire, SF-36: Short-Form Health Survey, HAD: Hospital Anxiety and Depression Scale.
When comparing changes between groups; in group 1 the average change in 6mWT was 40 (min 21, max 50) meters, while in group 2 the change was 50 (min 30, max 70) meters (Table 2). There was no significant difference between groups in terms of 6mWT change. The changes in PaO2 levels were significantly higher in group 2, compared to group 1 (Table 2). Improvement of dyspnea sensation was significantly higher in patients with severe diffusion defect compared to group 1 (p=0.04) (Table 2). There was no difference in SGRQ scores, SF-36 and HAD scores between groups 1 and 2 comparing changes in QoL parameters (p>0.05) (Table 2). In addition; FEV1% and DlCO increased in patients with only severe diffusion defect (p<0.05) (Table 2).
DiscussionIn this study we found significant improvement in oxygenation (PaO2), severity of dyspnea, exercise capacity and quality of life parameters in patents with moderate and severe diffusion defect after PR program. Additionally FEV1 and DlCO increased significantly in patients with severe diffusion defect. When comparing changes between groups; improvement in dyspnea sensation was significantly higher in patients with severe diffusion defect.
All studies about effects of pulmonary rehabilitation on lung function have investigated FEV1, FVC, FEV1/FVC and blood gas analysis. There are few studies conducted on investigating the effect of PR program on different DlCO groups.21,22 COPD is characterized by high morbidity and mortality. It remains unknown which aspect of lung function carries the most prognostic information. In a study of 604 COPD patients which were clinically stable it was concluded that DlCO was the most powerful predictor of survival.23 In another study in patients with COPD it was emphasized that FEV1, IC and DlCO were higher predictive regarding exercise capacity and DlCO was the strongest predictor.11 In a 5-year follow up study it was found that decline in 12-minute walk test was mainly explained by deterioration in DlCO and this measurements at baseline were the most important predictors of declining exercise capacity in COPD patients.24
Single measurements of DlCO in patients with COPD have shown that a reduced value in early disease is associated with accelerated decline in FEV1 and in advanced disease it predicts exercise capacity and mortality. In population studies a reduced DlCO predicts all-cause mortality more strongly than a reduced FEV1. It also stated that repeated measurements of CO transfer in individuals were needed to increase the present poor knowledge of the natural history of the contribution of alveolar disease to the progression of COPD.25 In a study performed in heavy smokers lower diffusing capacity was found to be directly correlated with decline FEV1/FVC ratio and a greater progression of CT – quantified emphysema.26 Mohsenifar et al. demonstrated that patients with reduced DlCO, particularly when <20% of predicted, were more likely to have reduced PaO2 at rest and were more likely to require supplemental oxygen with low levels of activity. They pointed out DlCO was useful in evaluating whether supplemental oxygen is required for exercise.27 In our study, consistent with the other studies, FEV1, FEV1/FVC, PaO2, SaO2, exercise capacity and quality of life scores were significantly lower in patients with severe diffusion defect before pulmonary rehabilitation.
Pulmonary rehabilitation has emerged as a recommended standard of care for patients with chronic lung disease.1,28 It has been demonstrated to improve exercise capacity, reduce symptoms of dyspnea and increase health-related quality of life.28,32 Pulmonary function tests showed different results after pulmonary rehabilitation in previous studies. In most of them, significant changes in FEV1, FVC and FEV1/FVC values5–8 were not detected. Because rehabilitation is a multicomponent intervention the results are controversial. In a study of 24 patients with COPD who carried out at least half an hour of pranayama breathing exercises for 3 months, despite significant increase in PEF values, there was no significant increase in FEV1 and FVC.33 Cecily et al. had observed that as well as FEV1 and FVC the value of PEFR (peak expiratory flow rate) significantly had increased in 100 patients with COPD.34 Shebl et al. concluded that FEV1 increased only in severe COPD but FVC and FEV1/FVC ratio was increased in the medium and severe COPD after the supervised two- month home based exercise program. However, these increases were not statistically significant.35 In a study comparing differences of improvement by gender, FEV1 and FVC increased in both; however they were greater in males after pulmonary rehabilitation program.36 When 225 patients were assessed according to severity of COPD; FEV1 increased significantly in stage 3 and 4, VC (vital capacity) increased significantly to 2.3 and 4, TLC (total lung capacity) decreased significantly only in stage 3, RV (residual volume) were significantly decreased in stage 3 and 4 after pulmonary rehabilitation program.37 Unlike other studies, after pulmonary rehabilitation for 3 years, there was not a significant fall in FEV1 at the end of the 3rd year.38
Changes in arterial blood gas were evaluated less and the results vary in previous studies. In some of the studies PaO2, PaCO2 and SaO2 did not change6,29 but in some other studies both PaO2 and SaO2 increased significantly after pulmonary rehabilitation.21–39 A study that assessed blood gas analysis according to severity of COPD showed that PaO2 increased significantly with stage 3 and 4, PaCO2 decreased significantly in stage 4 diseases after PR.37 There are few studies evaluating changes in diffusing capacity after PR. In a previous study conducted in our clinic there was a significant increase in DlCO level after 8 week outpatient rehabilitation program in 44 patients with COPD.21
Zanini et al. divided moderate to severe COPD patients (n: 75) into two groups depending on the change in 6MWT (responders >30m and no responders ≤30m). They showed that FEV1<50% pred. and TLCO<50% pred. were independent predictors of PR efficacy. They also found that complex COPD patients with poor lung function got more benefit from PR.39 Sixty patients were stratified into subgroups according to airway obstruction (FEV(1)>= or <50% predicted), pulmonary hyperinflation (TLC>or < or =120% predicted), BMI value (BMI > or < or =25), cardiovascular (CV) comorbidity, and resting PaO(2) (PaO(2)>= or <60mmHg) levels, suggesting that subjects with poorer exercise capacity or quality of life had greater room for improvement.40 An observational study, which included 102 COPD patients who followed PR showed that patients with worse disease status (i.e. a combination of lower FEV1, more hyperinflation, lower exercise capacity and worse quadriceps force) improved most in endurance exercise capacity.41 Our results were similar to the results of these studies; although it did not reach statistical difference, there was an improvement in the exercise capacity of the patients with severe diffusion defect when comparing with the patients having moderate diffusion defect. It is possible that the improvement in 6MWT partially reflects the gain in DLCO and lung function or simply reflects the fact that subjects with worse COPD have more room for improvement than those with mild COPD. It may also indicate that emphysematous COPD subjects have a better chance of improvement from rehabilitation than chronic bronchitis. In addition, PaO2 and O2 saturation values were significantly increased in both groups with a little difference in favor of the patients with severe diffusion defect. In COPD patients, exercise capacity may decrease as a result of exercise intolerance, ventilation and gas exchange impairment, cardiac failure and pulmonary muscle dysfunction or combination of them. Hypoxia due to gas exchange dysfunction may directly or indirectly decrease exercise tolerance.20 DlCO which is a good predictor of gas exchange dysfunction can also predict exercise restriction perfectly.11 Older textbooks suggest that thickening of the alveolar-capillary membrane (in interstitial lung disease) and loss of alveolar membrane surface area (in emphysema) are the primary causes of a low DLCO.42 However, new experimental data suggest that diseases that influence the DLCO do so by reducing the volume of red blood cells in the pulmonary capillaries.43 In healthy adults total volume of blood in lungs is less than 150ml at rest.43 Diseases which reduce alveolar-capillary surface area cause a reduction in the blood volume in the lungs. The blood volume in the pulmonary capillaries and the DLCO are increased during exercise.42 Therefore, we hypotheses that the increase in DLCO after PR may be due to the recruitment of pulmonary capillaries after exercise.
Patients with severe diffusion defects usually avoid exercise in daily life. However, PR program including aerobic and reinforcement exercise combination may improve their muscle power, exercise performance and quality of life, and may give them the courage to compete with their dyspnea symptom.44 Improvement in DlCO and FEV1 in this group of patients may reflect the success in muscle exercise as a result of recruitment of pulmonary capillaries. Also by breathing exercises it is possible that the exercise may reverse some dead space ventilation, and make new areas of alveolar more available to gas exchange.
Seventy-four stable COPD patients were grouped according to MMRC dyspnea scale and evaluated after PR. There was an improvement in 6-minute walking test and SGRQ only in the patients with dyspnea score: grade 1/2 and grade 3/4 while the improvement in grade 5 patients was very limited. The authors suggested that baseline state is a poor predictor of response to rehabilitation.45 Despite that, in our study, baseline DlCO levels were good predictors for better PR outcome. Although the MMRC and BORG scores were higher in patients with severe diffusion defect, these scores significantly decreased after PR. Also walking distance within group 2 (not statistically significant between groups) was increased significantly after PR. When the two groups were compared in terms of MMRC and BORG dyspnea score changes, the augmentation of MMRC and BORG score was significantly higher in patients with severe diffusion defect. Additionally, anxiety score was the only parameter decreased in patients with moderate diffusion defect whereas both anxiety score and depression score were decreased in patients with severe diffusion defect.
ConclusionWe concluded that pulmonary rehabilitation improves oxygenation, severity of dyspnea, exercise capacity and quality of life in patents with COPD independent of carbon monoxide diffusion capacity levels. Furthermore pulmonary rehabilitation may improve DlCO and FEV1 values in COPD patients with severe diffusion defect. However long-term follow up studies are needed to have the best results from rehabilitation programs.
The authors have no declarations of interest to report.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflict of interest.